On this page
Key information
|
Mode of transmission |
Faecal–oral route, either from person-to-person contact or through contaminated food or drink. It is also occasionally spread by injected drug use. |
|---|---|
|
Incubation period |
28–30 days average (range 15–50 days). |
|
Period of communicability |
The 1–2 weeks before and the first few days after the onset of jaundice. |
|
Burden of disease |
Infants and children are usually asymptomatic. Severity in adults increases with age. The disease is more serious in those with chronic liver disease and the immunocompromised. There is no carrier state. |
|
Funded vaccines |
Monovalent inactivated hepatitis A virus (HAV) vaccine: Havrix. |
|
Other available vaccines |
Monovalent inactivated hepatitis A virus (HAV) vaccine: Avaxim. Combined inactivated HAV-recombinant HBsAg protein vaccine: Twinrix. Combined HAV-purified Salmonella typhi Vi polysaccharide vaccine: Vivaxim. |
|
Dose, presentation, route |
Havrix 1440, Twinrix, Vivaxim: 1.0 mL per dose. Havrix Junior, Twinrix Junior, Avaxim: 0.5 mL per dose. Pre-filled syringe. Intramuscular injection. |
|
Funded vaccine indications and recommended schedule |
HepA vaccine (Havrix 1440) is recommended and funded for:
|
|
Recommended, unfunded |
Individuals working with children, exposed to faeces or contaminated water, or with non-human primates Armed forces personnel and travellers visiting high-risk countries Adults with chronic liver disease, including with chronic hepatitis B and C infection Men who have sex with men Food handlers during community outbreaks |
|
Vaccine efficacy |
High efficacy: HAV infection has been almost eliminated in immunised populations. |
|
Public health measures |
All cases of hepatitis A must be notified immediately on suspicion to local medical officer of health |
|
Post-exposure prophylaxis |
In an outbreak (if within 2 weeks of exposure):
|
8.1. Virology
Hepatitis A virus (HAV) is a ribonucleic acid (RNA) virus belonging to the picornavirus group, which also contains enteroviruses and rhinoviruses. The virus is usually transmitted by the faecal–oral route, either from person-to-person contact or through contaminated food or drink.
HAV primarily replicates in the liver and is excreted in large quantities via the biliary tract into the faeces. It is a hardy virus and can survive outside the body for prolonged periods in food and water. It causes a self-limiting illness with no carrier state.
8.2. Clinical features
The incubation period between ingestion of the virus and clinical symptoms is 15 to 50 days, with an average of 28 to 30 days. The virus can be detected in blood and faeces within a few days of ingestion, and it increases to a peak in the two weeks prior to the onset of clinical illness, which is the time that subjects are most likely to spread the infection. Faecal viral shedding continues for one to three weeks in adults, but has been reported to last longer in young children. Virus excretion falls sharply in the week following the onset of hepatitis.
In infants and preschool children, most infections are either asymptomatic or cause only mild, non-specific symptoms without jaundice. Most adults and adolescents develop symptomatic disease, the severity of which generally increases with age. Symptomatic HAV infection is characterised by an acute febrile illness with jaundice, anorexia, nausea, abdominal discomfort, malaise and dark urine. Signs and symptoms usually last less than two months, although 10–15 percent of symptomatic persons have prolonged or relapsing illness lasting up to six months. Liver enzymes almost always return to normal by six months after the illness, and often much sooner. The disease is more serious in people with chronic liver disease or those who are immunocompromised (including people with HIV infection). Chronic carrier states do not occur following hepatitis A infection and persisting liver damage is very rare.
8.3. Epidemiology
8.3.1. Global burden of disease
8.3.1. Global burden of disease
HAV is common in areas with poor sanitary conditions and limited access to clean water.[1] In highly endemic areas, such as parts of Africa and Asia, the disease is virtually confined to early childhood and is not an important cause of morbidity.[1, 2] Almost all adults in these areas are immune, and hepatitis A epidemics are uncommon. In intermediate endemicity areas, such as Central and South America, Eastern Europe and parts of Asia, children may not be infected in early childhood and reach adulthood without immunity. A high proportion of adolescents and adults are susceptible and large outbreaks are common. In low endemicity areas, such as the US and Western Europe, infection is less common but can occur in high-risk groups. Large outbreaks are rare, due to high levels of sanitation that stops person-to-person transmission.
Viral spread occurs readily in households, in early childhood services and in residential facilities that care for the chronically ill, disabled or those with a weakened immune system. In early childhood services, typically the adult guardian develops symptomatic disease while the primary source, the infected young child, is asymptomatic. The risk of spread in early childhood centres is proportional to the number of children aged under 2 years wearing nappies. Infection in these early childhood services is an important source of outbreaks for whole communities.
Other groups at the highest risk of contracting the disease include people in close contact with an infected person, and travellers to areas with high or intermediate rates of hepatitis A infection. Others also at greater risk of contracting HAV are people who have oral–anal sexual contact, illicit drug users, those with chronic liver disease, food handlers and laboratory staff working with the virus.
Universal and targeted programmes for childhood immunisation have been introduced in several countries, including Israel, the US and Australia. Acute HAV infection has almost been eradicated in areas with HAV immunisation programmes.
8.3.2. New Zealand epidemiology
8.3.2. New Zealand epidemiology
The rate of HAV in New Zealand declined from 145.7 per 100,000 in 1971 to 1.2 per 100,000 in 2019 (ESR, 8 June 2020).[3, 4] This fall in rate is attributable to the use of HAV vaccination in travellers and a reduction in HAV prevalence overseas.
In 2019, 58 cases were notified compared with 68 in 2018 (ESR, 8 June 2020). Hospitalisation status was recorded for 57 cases, of which, 36 (63 percent) were hospitalised.
The highest rates occurred in the 15–19 years (2.2 per 100,000) and 1–4- and 20–29-years age groups (both 2.0 per 100,000). Of the 56 cases with ethnicity information recorded, Pacific peoples had the highest notification rate (5.4 per 100,000), followed by the Asian (3.1 per 100,000) ethnic groups (ESR, 8 June 2020).
Overseas travel information was recorded for 55 cases: 32 cases (58.2 percent) had travelled overseas during the incubation period of the disease (ESR, 8 June 2020). The countries most frequently visited included India and Samoa (7 cases each), Fiji (5 cases), Indonesia (4 cases) and Tonga (3 cases). Four cases reported travel to more than one country.
Hepatitis A outbreaks continue to occur. There was one outbreak in 2019, involving 10 cases (ESR, 8 June 2020).
Figure 8.1 illustrates the overall national downward trend since a peak of notifications in 1997.
Figure 8.1: Hepatitis A notifications, by year, 1997–2019
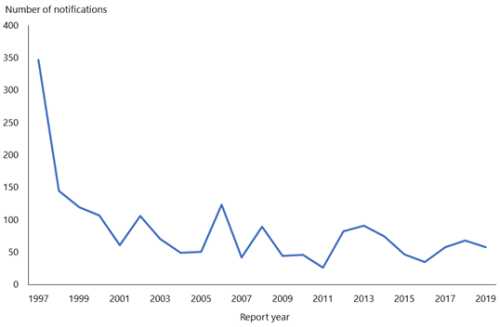


Source: ESR
For further details of hepatitis A notifications in New Zealand, refer to the most recent notifiable disease annual reports from ESR.
8.4. Vaccines
8.4.1. Available vaccines
8.4.1. Available vaccines
Two inactivated HAV vaccines (HepA) are currently registered (approved for use) and available (marketed) in New Zealand, as well as a combined HepA-HepB vaccine and a combined HepA-typhoid vaccine.
Funded vaccine
Hepatitis A vaccine (HepA) is not on the Schedule, but is recommended and funded for certain high-risk groups, as shown in Table 8.1.
Each 1.0 mL dose of Havrix 1440 (GSK) contains 1,440 EU (enzyme-linked immunosorbent assay units) of inactivated hepatitis A virus (HAV) adsorbed onto aluminium hydroxide. Each 0.5 mL dose of Havrix Junior contains 720 EU of inactivated HAV. Other components and residuals include neomycin sulphate, 2‑phenoxyethanol, polysorbate 20, amino acid supplement in a phosphate buffered saline solution.
Other vaccines
Inactivated HAV vaccine
Avaxim (Sanofi) contains 160 antigen units of inactivated HAV in each 0.5 mL dose; other components and residuals include aluminium hydroxide, phenoxyethanol, formaldehyde, Medium 199, neomycin and bovine serum albumin.
Combined HAV and HBV vaccine
Twinrix (GSK) contains 720 EU of inactivated HAV and 20 µg of recombinant DNA HBsAg vaccine in each 1.0 mL dose. The Twinrix Junior preparation (0.5 mL per dose) contains half these amounts. The vaccines are adsorbed onto aluminium adjuvants. Other components and residuals include aluminium hydroxide, aluminium phosphate, sodium chloride, amino acids, dibasic sodium phosphate, formaldehyde, monobasic sodium phosphate, neomycin sulphate, polysorbate 20 and trometamol.
Combined HAV and typhoid vaccines
Vivaxim (Sanofi) contains 160 antigen units of inactivated HAV and 25 µg of purified Salmonella typhi Vi polysaccharide in each 1.0 mL dose; other components and residuals include sodium chloride, sodium phosphate, aluminium hydroxide, phenoxyethanol, formaldehyde, Medium 199, neomycin and bovine serum albumin.
8.4.2. Efficacy and effectiveness
8.4.2. Efficacy and effectiveness
After one dose of monovalent HepA in healthy people, protective levels of antibody have been demonstrated by two weeks, and 94–100 percent of people vaccinated will seroconvert by four weeks.[5]
A second dose 6 to 18 months after the first is thought to be important for long-term protection, particularly in the absence of exposure to HAV.[6,7] In subjects with an impaired immune system, adequate anti-HAV antibody titres may not be obtained after a single dose.
Hep A vaccines have not yet been approved for children aged under 12 months. This is due to the potential interference from maternal antibody which may affect long term immunity.[8] However, HepA vaccines have been shown to be safe and efficacious in infants as young as 2 months.[9] Such that the CDC recommends HepA vaccination for infants aged 6–11 months travelling outside of the US.[10]
HepA vaccines are highly effective in preventing clinical disease, with recorded efficacy measures of around 94–100 percent from six weeks post-vaccination. Where children, adolescents and young adults have been vaccinated in targeted and/or national programmes, there has been a rapid decline in disease incidence. This decline is through both direct and indirect (herd immunity) effects.[6]
Duration of immunity
Antibodies to two doses of HepA have been shown to persist in vaccinated adults for at least 17 years after vaccination, and up to 15 years in vaccinated children and adolescents.[11] Mathematical models estimate that following completion of a two-dose series, protective levels of antibody persist for 40 years or longer in adults and 14–20 years in children.[11] Given that HAV has a long incubation period, it is possible that immune memory with no detectable circulating antibody may be sufficient for protection, as is the case with HBV and HepB.
8.4.3. Transport, storage and handling
8.4.3. Transport, storage and handling
Transport according to the National Standards for Vaccine Storage and Transportation for Immunisation Providers 2017 (2nd edition).
Store at +2°C to +8°C. Do not freeze.
8.4.4. Dosage and administration
8.4.4. Dosage and administration
See Table 8.2 for dosage and scheduling information.
The monovalent HepA and HepA combination vaccines should be administered by intramuscular injection into the deltoid region of the upper arm in adults and older children, or the anterolateral aspect of the thigh in younger children (see section 2.2.3).
Co-administration with other vaccines
The monovalent HepA and HepA-combination vaccines may be administered concurrently with other vaccines.[11, 12] The vaccines should be given in separate syringes and at different injection sites.
Interchangeability of hepatitis A vaccines
The monovalent HepA vaccines may be used interchangeably to complete a two-dose course.[12]
8.5. Recommended immunisation schedule
Table 8.1: Hepatitis A vaccine recommendations
Table 8.1: Hepatitis A vaccine recommendations
| Note: Funded individuals are in shaded rows. See the Pharmaceutical Schedule for any changes to the funding decisions. |
|
Recommended and funded |
|---|
|
Transplant patientsa |
|
Children with chronic liver diseasea |
|
Close contactsb of hepatitis A cases |
|
Recommended but not funded |
|
Adults with chronic liver disease:
|
|
Men who have sex with men |
|
Travellers – including occupationalc and recreational travel |
|
Occupational groupsc exposed to faeces, including:
|
|
Food handlersc during community outbreaks. |
|
Armed forces personnelc who are likely to be deployed to high-risk areas. |
|
a. See also section 4.3.10. b. Only one dose is funded for close contacts as protection is only required for the duration of the outbreak. For long-term protection, contacts may seek a second (unfunded) dose, after an interval of at least 6 months. See the Hepatitis A chapter of the Communicable Disease Control Manual for a definition of contacts. c. May be employer-funded. See also section 4.8. |
8.5.1. Recommendations
8.5.1. Recommendations
Hepatitis A vaccines are not on the Schedule, but are funded for the high-risk groups as shown in the shaded section of Table 8.1. They may also be employer-funded or funded during an outbreak (see section 8.8).
Individuals with chronic liver disease
HepA is recommended and funded for children with chronic liver disease and for children and adults undergoing transplants (see sections 4.3.11 and 4.5). People with chronic liver disease are not at increased risk for hepatitis A, but acute hepatitis A can have serious or fatal consequences.[6]
Chronic hepatitis B or C infection
Studies have shown that in these individuals, super-infection with HAV leads to increased morbidity and mortality.[6]
Other chronic liver disease
Non-immune individuals who have not been vaccinated should receive HepA before liver decompensation. It should be given as early as possible before liver transplantation; vaccination may be performed after transplantation, although the response is unlikely to be as good as early in liver disease.[13, 14]
Travellers
The first dose of HepA should be given as soon as travel is considered.[11] The high and intermediate endemicity areas listed in section 8.3.1 may be used as a guide for recommending hepatitis A vaccination for travel, but there are limits to the data that informs these listings, and variation within countries. Even in low prevalence countries there is a risk of foodborne hepatitis A. In addition, decreasing prevalence in formerly endemic countries leads to large numbers of susceptible people and the risk of large outbreaks, as has recently been reported. The vaccine may be considered for all travellers. Although licensed from age 1 year, HepA could also be considered for use in infants younger than 1 year if at significant risk of infection.[10]
Immunoglobulin is not normally available or recommended in New Zealand for pre‑travel use.
Certain occupational groups
Immunisation with HepA is recommended (but not funded) for people in occupational groups exposed to faeces, as listed in Table 8.1 above.
Others at higher risk
Pre-immunisation screening for anti-HAV antibodies is not routinely recommended. There is no danger in vaccinating an already immune person, but some groups with higher probability of prior infection may wish to avoid the expense of vaccination. These include:
- those who are likely to have been exposed as children (born in a country of high endemicity) or in the course of their employment
- those with a history of jaundice.
Consider HepA for the following groups:
- intravenous drug users (who account for 30 percent of cases in communities during outbreaks)[6]
- men who have sex with men.
Routine immunisation for children
HepA is not routinely recommended and is not on the Schedule for children in New Zealand. It should, however, be considered during community outbreaks (see section 8.8).
8.5.2. Immunisation schedule
8.5.2. Immunisation schedule
Immunisation schedules for HAV-containing vaccines are provided in Table 8.2. See the manufacturers’ data sheets for more information. For monovalent HepA, the first dose is for primary immunisation and the second dose is a booster.
Table 8.2: Hepatitis A-containing vaccines: by age, dose and schedule
| Note: Havrix 1440 and Havrix Junior are funded for eligible individualsa (see Table 8.1). |
|
Age |
Vaccine |
Dose |
Volume (mL) |
Number of doses |
Schedule |
|---|---|---|---|---|---|
|
Hepatitis A vaccines |
|||||
|
1–15 years |
Havrix Juniora |
720 EU |
0.5 |
2 |
0 and 6–12 monthsb |
|
2 years–adult |
Avaxim |
160 antigen units |
0.5 |
2 |
0 and 6–36 months |
|
≥16 years |
Havrix 1440a |
1,440 EU |
1 |
2 |
0 and 6–12 monthsb |
|
Hepatitis A–Hepatitis B combined vaccine |
|||||
|
1–15 years |
Twinrixc |
720 EU of HAV and 20 µg of HBsAg |
1.0 |
2 |
0 and 6–12 months |
|
Twinrix Juniord |
360 EU of HAV and 10 µg of HBsAg |
0.5 |
3 |
0, 1 and 6 months |
|
|
≥16 years |
Twinrix |
720 EU of HAV and 20 µg of HBsAg |
1.0 |
3 |
0, 1 and 6 months; or 0, 7, and 21 days plus a booster at 1 year |
|
Hepatitis A–Typhoid combined vaccines |
|||||
|
≥16 years |
Vivaxim |
160 antigen units of HAV and 25 µg of Vi |
1.0 |
1 |
At least 14 days before departure; then boost with HepA at 6–36 monthse |
|
Key: EU = enzyme-linked immunosorbent assay (ELISA) units of hepatitis A virus protein; HAV = hepatitis A virus; HBsAg = recombinant hepatitis B surface antigen; Vi = Salmonella typhi polysaccharide
a. Note that two doses of HepA are funded for transplant patients and children with chronic liver disease (see sections 4.3.11 and 4.5); one dose is funded for close contacts of hepatitis A cases. b. Even after a longer interval between the first and second doses, there is no need to restart the series. A substantial anamnestic response occurs after a second dose given up to 8 years after the initial dose.[16] c. For children not previously exposed to the hepatitis A or B viruses. Source: GlaxoSmithKline NZ Ltd. 2016. Twinrix and Twinrix Junior New Zealand Data Sheet [PDF, 85 KB] (accessed 19 June 2020). d. Use when the child is at immediate risk of exposure to hepatitis B (eg, travellers) and did not receive a primary course of HepB as an infant. Source: GlaxoSmithKline NZ Ltd. 2019. Twinrix and Twinrix Junior New Zealand Data Sheet [PDF, 85 KB] (accessed 19 June 2020). e. If the individual remains at risk from typhoid fever, a single dose of the typhoid vaccine is recommended every 3 years. |
|||||
8.5.3. Pregnancy and breastfeeding
8.5.3. Pregnancy and breastfeeding
The safety of HepA during pregnancy and while breastfeeding has not been determined. However, because HepA is produced from inactivated HAV, there is not expected to be any risk to the developing fetus and infant. As a precaution, HepA should be used during pregnancy only when clearly needed, such as when travelling to a country where HAV is endemic.
8.6. Contraindications and precautions
See also section 2.1.3 for pre-vaccination screening guidelines and section 2.1.4 for general contraindications for all vaccines.
8.6.1. Contraindications
8.6.1. Contraindications
Administration of HepA should be delayed in individuals suffering from acute febrile illness. HepA should not be administered to people with a history of an anaphylactic reaction to a prior dose of HepA or to a vaccine component.
8.6.2. Precautions
8.6.2. Precautions
In individuals with an impaired immune system, adequate anti-HAV antibody titres may not be obtained after a single dose.
Pregnancy is a precaution – see section 8.5.3.
8.7. Potential responses and AEFIs
8.7.1. Potential responses
8.7.1. Potential responses
Soreness, redness and swelling at the injection site; fever; malaise; headache; nausea; and loss of appetite have been reported for monovalent HepA, but these responses are usually mild and brief.[16] Similar responses are seen with HepA–HepB combination vaccines, and HepA–typhoid combination vaccines.
8.7.2. AEFIs
8.7.2. AEFIs
Review of data from multiple sources has not identified any serious adverse events among children and adults that could be attributed to HepA.[16]
8.8. Public health measures
It is a legal requirement that all cases of hepatitis A be notified immediately on suspicion to the local medical officer of health.
For further details on public health control measures, see the Communicable Disease Control Manual.
8.8.1. Post-exposure prophylaxis and outbreak control
8.8.1. Post-exposure prophylaxis and outbreak control
Vaccination
Age-appropriate vaccine is recommended for all close contacts aged older than 1 year. If time allows, consider pre-vaccine serology if there is a history or likelihood of previous HepA vaccination or infection (eg, previous residence in an endemic country). Post-exposure prophylaxis with vaccine should be offered to contacts as soon as possible, and within two weeks of last exposure to an infectious case. The efficacy of vaccine when administered more than two weeks after exposure has not been established.
Immunoglobulin
Where vaccine is contraindicated (or not immediately available), human normal immunoglobulin may be offered to a close contact who may have a reduced response to vaccine or has risk factors for severe disease. The dose is 0.03 mL/kg given by intramuscular injection. Post-exposure prophylaxis should be offered to contacts as soon as possible, and within two weeks of last exposure to an infectious case.
For post-exposure prophylaxis, close contacts aged under 1 year may require human normal immunoglobulin. This should be discussed with the appropriate infectious disease physician.
Human normal immunoglobulin is available from the New Zealand Blood Service. For further information, see the medicine data sheets or the New Zealand Blood Service website.
Early childhood services and other institutional outbreaks
If an outbreak occurs in an early childhood service, vaccination (and/or immunoglobulin if appropriate) may be indicated for all previously unimmunised staff and children at the service and unimmunised new staff and children for up to six weeks after the last case has been identified, including cases in the household of attendees.
For more details on control measures, see the ‘Hepatitis A’ chapter of the Communicable Disease Control Manual.
8.9. Variations from the vaccine data sheets
Havrix Junior is licensed from ages 1 to 15 years of age. However, for infants travelling to areas at high risk of hepatitis A infection and pre-exposure prophylaxis, Health New Zealand recommends that HepA vaccination (Havrix Junior) can be given under 1 year of age at least two weeks prior to departure.
References
References
References
- Nelson N ,Murphy T. 2016. Hepatitis A. in CDC Health Information for International Travel (Yellow Book), Brunette GW (ed) (eds). URL: https://wwwnc.cdc.gov/travel/yellowbook/2020/travel-related-infectious-diseases/hepatitis-a. (accessed 10 May 2022)
- World Health Organization. Hepatitis A Factsheet. [updated 21 July 2021]; URL: https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-a. (accessed 10 May 2022)
- Institute of Environmental Science and Research Ltd. 2016. Notifiable Diseases in New Zealand: Annual Report 2015 (ed.), Porirua, New Zealand: The Institute of Science and Environmental Research Ltd. URL: https://surv.esr.cri.nz/PDF_surveillance/AnnualRpt/AnnualSurv/2015/2015AnnualReportFinal.pdf (accessed 3 July 2020)
- Institute of Environmental Science and Research Ltd. 2019 Notifiable Diseases in New Zealand: Annual Report 2017. Porirua, New Zealand. URL: https://surv.esr.cri.nz/PDF_surveillance/AnnualRpt/AnnualSurv/2017/2017AnnualNDReport_FINAL.pdf. (accessed 3 July 2020)
- Centers for Disease Control and Prevention. 2006. Prevention of Hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report: Recommendations and Reports. 55(RR07): p. 1–23. URL: www.cdc.gov/mmwr/PDF/rr/rr5507.pdf (accessed 3 July 2020)
- Averhoff F, Khudyakov Y ,Nelson N. 2018. Hepatitis A vaccines, in Plotkin's Vaccines (7th Edition), Plotkin S, Orenstein W, Offit P, and Edwards K (eds). Elsevier: Philadelphia, US.
- Van Damme P, Banatvala J, Fay O, et al. Hepatitis A booster vaccination: is there a need? Lancet, 2003. 362(9389): p. 1065-71.
- Bell BP, Negus S, Fiore AE, et al. Immunogenicity of an inactivated hepatitis A vaccine in infants and young children. Pediatric Infectious Disease Journal, 2007. 26(2): p. 116-22.
- Dagan R, Amir J, Mijalovsky A, et al. Immunization against hepatitis A in the first year of life: priming despite the presence of maternal antibody. Pediatric Infectious Disease Journal, 2000. 19(11): p. 1045-52.
- Nelson NP, Link-Gelles R, Hofmeister MG, et al. Update: Recommendations of the Advisory Committee on Immunization Practices for Use of Hepatitis A Vaccine for Postexposure Prophylaxis and for Preexposure Prophylaxis for International Travel. MMWR: Morbidity and Mortality Weekly Report, 2018. 67(43): p. 1216-1220.
- American Academy of Pediatrics. 2018. Hepatitis A. in Red Book: 2018 Report of the Committee on Infectious Diseases, Kimberlin D, Brady M, Jackson M, and Long S (eds). URL: https://redbook.solutions.aap.org/redbook.aspx. (accessed 3 July 2020)
- Australian Technical Advisory Group on Immunisation (ATAGI). 2018. Hepatitis A. in Australian Immunisation Handbook. Canberra. URL: https://immunisationhandbook.health.gov.au/vaccine-preventable-diseases/hepatitis-a. (accessed 25 May 2020)
- Arslan M, Wiesner RH, Poterucha JJ,Zein NN. Safety and efficacy of hepatitis A vaccination in liver transplantation recipients. Transplantation, 2001. 72(2): p. 272-6.
- Arguedas MR, Johnson A, Eloubeidi MA,Fallon MB. Immunogenicity of hepatitis A vaccination in decompensated cirrhotic patients. Hepatology, 2001. 34(1): p. 28-31.
- Iwarson S, Lindh M ,Widerstrom L. Excellent booster response 4 to 8 years after a single primary dose of an inactivated hepatitis A vaccine. Journal of Travel Medicine, 2004. 11(2): p. 120-1.
- Irving GJ, Holden J, Yang R,Pope D. Hepatitis A immunisation in persons not previously exposed to hepatitis A. Cochrane Database Syst Rev, 2012. CD009051.(7).