On this page
Key information
|
Mode of transmission |
Airborne droplets from, or contact with, vesicular lesions or possibly respiratory secretions. |
|---|---|
|
Incubation period |
Usually 14–16 days (range 10–21 days). |
|
Period of communicability |
From 2 days before onset of the rash until all lesions have crusted. |
|
Incidence and burden of disease |
Without immunisation, most people have infection during childhood. Groups at risk of severe complications include pregnant women and their unborn babies, and immunocompromised individuals. |
|
Funded vaccine |
VV (Varilrix) is a live attenuated vaccine. |
|
Dose, presentation, route |
0.5 mL per dose after reconstitution. Pre-filled syringe and glass vial. The vaccine must be reconstituted prior to injection. Intramuscular or subcutaneous injection. |
|
Funded vaccine indications and schedule |
1 dose is funded for:
Up to 2 doses are funded for certain special groups and their household members if non-immune to varicella. |
|
Recommended |
Susceptible children and adults who are not eligible by age for funded vaccine. |
|
Vaccine effectiveness |
One dose confers approximately 99% protection against severe disease and 80% protection against varicella disease of any severity. Breakthrough disease is usually mild. Herd immunity has been documented. |
|
Contraindications |
Pregnancy Certain immune deficiency and immunocompromised states Known anaphylaxis to neomycin, gelatin or other vaccine components Active untreated TB (see section 24.6) |
|
Potential responses to vaccine |
Generally mild and self-limiting, and include local reactions, fever and mild papulo-vesicular rash in normal healthy individuals. |
|
Post-exposure prophylaxis |
VV may be used for post-exposure prophylaxis for immune-competent people if given within 5 days of exposure. Zoster immunoglobulin (ZIG) is most effective if given as soon as possible after exposure but may be given up to 10 days post-exposure (see section 24.8.2). |
24.1. Virology
Varicella (chickenpox) is a highly infectious disease caused by human herpes virus type 3 (varicella zoster virus or VZV). Reactivation of latent VZV results in herpes zoster (zoster; shingles), a disease with considerable morbidity (see chapter 25).
24.2. Clinical features
Varicella is one of the most infectious diseases known (along with pertussis and measles). Transmission occurs via airborne droplets from, or contact with, vesicular lesions and possibly respiratory tract secretions. The incubation period is usually 14–16 days (range 10–21 days but can be longer in immunocompromised individuals and those who have received ZIG), and cases are infectious from two days before the onset of the rash until all the lesions have crusted. A maculopapular rash, which becomes vesicular, appears in crops over several days, first on the face and scalp, later spreading to the trunk and then the limbs. Vesicles, ranging in number from few to many hundred, dry and crust after three to four days. A hallmark of the rash is lesions in varying stages of development. Lesions on mucosal surfaces (mouth, vagina) can cause considerable distress. The rash is pruritic and is usually associated with mild fever, malaise, anorexia and listlessness.
In most children, varicella is a mild disease but complications requiring hospitalisation and fatalities do occur. Secondary bacterial skin infections are common. Serious complications include central nervous system involvement (encephalitis, cerebellar ataxia, stroke), pneumonia, secondary invasive bacterial infections, and even death. Adults are 25 times more likely to develop severe disease than children, with pneumonia being the most common complication, often requiring mechanical ventilation. VZV pneumonia carries an overall mortality rate of 10–30 percent.
Maternal varicella occurring in the first half of pregnancy can cause the rare but devastating congenital varicella syndrome (see Table 24.4), whereas disease very late in pregnancy (from five days before to two days after delivery) may cause severe neonatal varicella infection. Pregnant women who contract varicella have an estimated 10–20 percent risk of developing VZV pneumonia.
Others vulnerable to both VZV and zoster are those who are immunocompromised, such as people taking immunosuppressive medications (eg, cancer treatment or organ transplant patients) and those with HIV infection. Varicella can be a fatal disease in immunocompromised individuals.
VZV infection is followed by the production of VZV-specific T-cell mediated immunity, necessary to maintain the latency of VZV in the ganglia and prevent reactivation as zoster. The immune response is boosted by subclinical reactivation of latent virus. The incidence of zoster increases with age as VZV-specific T cell-mediated immunity declines (see chapter 25).
24.3. Epidemiology
24.3.1. Global burden of disease
24.3.1. Global burden of disease
In temperate climates, winter-spring epidemics occur with peak incidence in preschool and early primary school ages (1–9 years). Around 90 percent of individuals have been infected by adolescence and fewer than 5 percent of adults are susceptible. The annual number of infections therefore approximates the birth cohort.[1, 2]
Transmission of the virus is less efficient in tropical climates. Adolescent and adult immigrants to New Zealand from such countries are more likely to be susceptible, placing them at risk of contracting chickenpox in their new environment. Being older, they are more likely to suffer severe disease.
The long incubation and high transmissibility of VZV conspire to maximise disruption to families: by the time the rash occurs a child will have been infectious for two days; any susceptible household members will then become unwell just as the first child recovers. This results not only in morbidity but also in financial consequences for parents missing work.
Crude hospitalisation admission rates in high-income countries range from around 2–6 per 100,000 population-year. Most of these admissions are children, consistent with the high incidence of varicella in children. Crude mortality rates ranged from 0.3–0.5 per million population-year with overall case fatality ratios of around 2–4 per 100,000 cases. Almost 90 percent of varicella hospital admissions occur in otherwise healthy and immunocompetent individuals.[1, 2]
VV has been introduced into childhood immunisation programmes overseas, including the US from 1995 and Australia from 2005, resulting in dramatic reductions in varicella morbidity, hospitalisations and mortality.[3, 4, 5] By 2005 in the US, vaccine coverage was approximately 90 percent and varicella incidence had declined by more than 90 percent. Herd immunity was observed outside of age groups targeted for vaccination.[1]
Following the introduction of VV onto the childhood schedule, exposure to wild-type virus decreases. It has been theorised that a lack of boosting may lead to an increase in zoster in older adults. However, studies that have investigated this issue have been unable to attribute any increase in incidence of zoster to the childhood VZV vaccine programme.[6, 7] Studies from the UK and Canada reported increases in zoster not associated with a vaccination programme, and some US data showed zoster rates were increasing prior to the initiation of their varicella vaccination programme.[8, 9]
24.3.2. New Zealand epidemiology
24.3.2. New Zealand epidemiology
Varicella is not a notifiable disease, so data is limited for uncomplicated varicella, but the epidemiology is likely to be as described above for temperate climates. With increasing participation in early childhood services, a greater proportion of infections may now be occurring in preschool-aged children. It is expected that zoster numbers will rise in New Zealand as the population ages.
Prospective nationwide surveillance of varicella in New Zealand children, conducted from November 2011 to October 2013, found that the incidence of varicella-related hospitalisation was 8.3 per 100,000 children per year – although this is likely to be a significant underestimate.[10] Māori and Pacific children were disproportionately affected, with an almost three- and four-fold increase in the relative risk of hospitalisation for varicella or its complications, respectively. Of the hospitalised children, 9 percent required ICU admission and most of them were previously healthy. Almost one-third of hospitalised children had multiple complications from varicella, and those with neurological complications were more likely to have ongoing problems at discharge.
A retrospective survey of admissions to the paediatric intensive care unit at Auckland’s Starship Children’s Hospital during 2001–2011 found 26 children admitted for varicella or its secondary complications.[11] The main admission reasons were neurological (38.5 percent) and secondary bacterial sepsis or shock (26.9 percent). Four children died (15 percent), three of whom were immunocompromised. A further eight children (31 percent) had ongoing disability at discharge, most having had no prior medical condition.
Based on overseas rates, it is estimated that up to one case of congenital varicella syndrome may be expected in New Zealand each year, although few have been reported.
In 2017, adults (aged 20 years and older) accounted for 25 percent of varicella related hospital admissions;[12] approximately one person per year dies from VZV infection, and most VZV-related deaths occur in adults.[13]
24.4. Vaccines
24.4.1. Available vaccines
24.4.1. Available vaccines
There are two live attenuated monovalent VVs registered (approved for use) and available (marketed) in New Zealand. Two quadrivalent live attenuated MMRV vaccines are registered but not currently available in New Zealand.
Funded vaccine
Monovalent VV
Varilrix, (GSK): each 0.5 mL dose contains no less than 103.3 PFU (plaque-forming units) of the varicella virus (VZV Oka strain). Other components include amino acids, lactose monohydrate and polyalcohols (mannitol and sorbitol) and residual neomycin sulphate.
Other vaccines
Monovalent VV (Varivax, MSD) contains not less than 1,350 PFU of the varicella-zoster virus (Oka/Merck strain). Other components and residuals include sucrose, gelatin, urea, sodium chloride, monosodium L-glutamate, potassium chloride, MRC-5 cells, neomycin and bovine calf serum. This vaccine is not currently available in New Zealand.
Quadrivalent MMRV is not available in New Zealand
- Priorix-Tetra (GSK) contains the Schwarz measles, RIT 4385 mumps, Wistar RA 27/3 rubella and varicella (Oka/Merck VZV) virus strains.
- ProQuad (MSD) contains Enders’ attenuated Edmonston (Moraten) measles virus strain, Wistar RA 27/3 rubella virus, Jeryl Lynn mumps virus and live varicella virus vaccines (Oka/Merck VZV)
See also section 12.4.1 for more information about MMR vaccines.
24.4.2. Efficacy and effectiveness
24.4.2. Efficacy and effectiveness
Single-dose varicella vaccination programmes have had a dramatic impact on the incidence of VZV infections,[14, 15, 16] hospitalisations [3, 4, 17] and serious outcomes,[5] particularly when high coverage rates are achieved. Indirect effects are also apparent. A 2014 systematic review of varicella vaccines found that a single dose of VV is moderately effective for preventing any severity of varicella (approximately 80 percent) in immune-competent individuals, highly effective for preventing moderate-severe disease (approximately 95 percent) and highly effective in preventing severe disease only (approximately 99 percent).[18] However, single-dose programmes are associated with outbreaks even among highly vaccinated groups.[19, 20]
The use of a second dose during outbreaks has been an effective strategy to prevent further cases. Catch‑ups for non-immunised groups without a previous history of varicella are also important. There is a significant reduction in breakthrough disease when two doses are given. After a second dose in children the immune response is markedly enhanced, with over 99 percent of children attaining an immune response thought to provide protection, and the geometric mean antibody titre is also significantly increased.
Over a 10-year period the estimated vaccine efficacy of two doses for prevention of any varicella disease is 98 percent (compared to 94 percent for a single dose), with 100 percent efficacy for the prevention of severe varicella. The likelihood of breakthrough varicella is reduced by a factor of 3.3.[21, 22] Because of this data, in 2006 the US authorities recommended a two‑dose strategy for varicella prevention, with the first dose at age 12–15 months and the second at age 4–6 years, as for MMR.[19, 21] A Hong Kong study recommended reducing the time between doses, to age 12 months and 18 months, to reduce breakthrough cases and outbreaks in preschools.[23]
The antigenic components of MMRV vaccines are non-inferior compared with simultaneous administration of MMR and VV,[24, 25] for both the first and second doses.
Herd immunity
In regions where universal varicella vaccination programmes have been implemented, significant declines in varicella cases and hospitalisation have been observed. These programmes also reduce circulating VZV and provide protection through herd immunity for those who are unable to be immunised, such as infants and immunocompromised individuals.
In the US, the annual average age-adjusted mortality rate for varicella was 0.05 per million population during 2008–2011, an 87 percent reduction from the pre-vaccine years.[26] In Canada between 2000 and 2007, a single dose of VV was introduced to the immunisation schedules of different provinces at 12 months of age; most provinces also included catch-ups for susceptible children at preschool or school. An ecological study of varicella-related hospitalisations in Canada between 1990 and 2010 found that hospitalisation rates decreased in all age groups, including infants and those aged 20–39 years.[27] Similar herd effects were seen in Germany, with declines in varicella cases and hospitalisations in infants and adolescents who were not eligible for VV.[28]
Duration of immunity
Varicella vaccination provides long-term but probably not lifelong immunity against VZV, in contrast to VZV natural infection. The duration of protection after a single dose of vaccine is difficult to study – especially if wild-type varicella continues to circulate liberally in the community, providing natural boosting and prolonging the duration of protection.[18] Many countries that initially introduced a single-dose vaccination programme have subsequently changed to a two-dose programme as the epidemiology of the disease has changed over time.
24.4.3. Transport, storage and handling
24.4.3. Transport, storage and handling
Transport according to the National Standards for Vaccine Storage and Transportation for Immunisation Providers 2017 (2nd edition).
VV requires reconstitution with the supplied diluent before administration.
Varilrix is presented as a lyophilised powder for reconstitution with the supplied diluent. The vaccine should be stored in the refrigerator at +2°C to +8°C, although the diluent may be stored at room temperature (to a maximum of 25°C). Reconstituted vaccine should be used immediately, if possible, and discarded if reconstituted vaccine is not used within 90 minutes (1½ hours) at room temperature. Do not freeze reconstituted vaccine.[29]
24.4.4. Dosage and administration
24.4.4. Dosage and administration
The dose of monovalent VV is 0.5 mL, administered by intramuscular or subcutaneous injection in the deltoid area (see section 2.2.3).
Co-administration with other vaccines
Monovalent VV can be administered concurrently with other vaccines, but in a separate syringe and at a different site. If not administered concurrently, the vaccine must be separated from other live vaccines (eg, MMR, BCG) by at least four weeks.
24.5. Recommended immunisation schedule
Recommendations for VV are summarised in Table 24.1 and discussed below.
VV is recommended to be administered with the second MMR dose and Hib-PRP at age 15 months. VV can be used for a second (unfunded) dose or first dose after age 4 years, as appropriate for those not in eligible special groups.
Table 24.1: Varicella vaccine recommendations and schedule
Table 24.1: Varicella vaccine recommendations and schedule
| Funded individuals are shown in shaded boxes. See the Pharmaceutical Schedule for any changes to funding decisions. |
|
Recommended and funded |
|---|
|
1 dose of VV for:
|
|
2 doses of VV, at least 6 weeks apart, for the following special groupsa
|
|
Recommended, not funded |
|
1 dose for all susceptible healthy children aged under 13 years who do not meet the eligibility criteria for the funded dose. 2 doses, at least 6 weeks apart, for all susceptible adolescents and adults. |
|
a. See chapter 4 ‘Immunisation of special groups’ for more information. b. See Table 4.4 for an accelerated immunisation schedule for infants in whom liver or kidney transplant is likely. d. Note that immunosuppression due to steroid or other immunosuppressive therapy must be for a treatment period of greater than 28 days. |
24.5.1. Usual childhood schedule
24.5.1. Usual childhood schedule
From 1 July 2020, one dose of VV is funded for children at age 15 months. A second VV dose is not currently funded but may be purchased for those who wish to reduce the risk of breakthrough disease.
A catch-up dose of VV is funded for previously unvaccinated children turning 11 years old on or after 1 July 2017, who have not previously had a varicella infection (as determined by clinical history). This dose aims to protect those who have not become immune to varicella before adolescence, as disease in adolescents and adults can be more severe.
Regardless of clinical history of varicella, children are recommended to receive one dose of VV at age 15 months (or as part of a catch-up immunisation) due to a risk of misdiagnosis of varicella, particularly in young children with a mild infection. There is no harm to vaccinate if they have previously had varicella. Serology is not routinely suggested.
24.5.2. Special groups
24.5.2. Special groups
Two doses of VV at least six weeks apart, are recommended and funded for the special groups listed in Table 24.1 above from age 9 months.
Immunocompromised (including immunosuppressed) individuals
The vaccine should not be given to immunocompromised individuals except under the direction and care of a specialist, following a suitable protocol[19] (see section 4.3).
Some clinical trials of the original vaccine formulations were conducted in immunocompromised children (with leukaemia in remission or with HIV infection on antiretroviral treatment). One study found that half the vaccinated children receiving maintenance chemotherapy developed a rash up to one month after vaccination (40 percent of these required acyclovir treatment), compared with 5 percent of those no longer on chemotherapy.[30] Despite this, the study concluded that the vaccine, Varivax, was safe, immunogenic and effective in these children.[30, 31]
Varicella immunisation of children with congenital T‑cell immune deficiency syndromes is generally contraindicated, but those with impaired humoral immunity may be immunised (see section 24.6.1 for further contraindications). Seek specialist advice.
Household contacts of immunocompromised individuals
Immunocompromised individuals are at highest risk of severe varicella and zoster infections.
Where such individuals cannot be vaccinated, it is important to vaccinate the non-immune household members and other close contacts (funded for household contacts) to provide ‘ring-fence’ protection (see sections 4.2, 4.3 and 24.7.1).
24.5.3. Recommended but not funded
24.5.3. Recommended but not funded
A single dose of VV is recommended for susceptible children who do not meet the eligibility criteria for funded vaccine (Table 24.1). A second dose may also be purchased for those who wish to reduce the risk of breakthrough disease.
VV in a two-dose schedule is recommended but not funded for the following groups:[19]
- adults and adolescents who were born and resided in tropical countries, if they have no history of varicella infection
- susceptible adults and adolescents (ie, those who have no prior history of chickenpox)
- susceptible individuals who live or work in environments where transmission of VZV is likely (see Table 4.9 for occupational groups)
- susceptible non-pregnant women of childbearing age
- susceptible international travellers[19]
- health care workers (see below)
- susceptible individuals who have been exposed to varicella (see section 24.8.3).
See section 24.8.1 for information about assessing susceptibility.
Health care workers
All health care workers should be immunised with VV if they are susceptible to varicella (not funded), particularly those within obstetric, paediatric and neonatal units, and those caring for immunocompromised children and adults. When a health care worker has a good history of prior varicella infection, no blood test is required.[32] If there is not a good history of varicella infection, a blood test to assess susceptibility will be necessary, as many individuals with no clinical history of varicella are immune (see section 24.8).
If a health care worker who has clinical contact with patients develops a rash as a result of the vaccine (around 5 percent), they must be excluded from contact with immunocompromised or other at-risk patients and allocated other duties, or excluded from their place of work, for the duration of the rash.
24.5.4. Pregnancy and breastfeeding
24.5.4. Pregnancy and breastfeeding
Varicella vaccines are contraindicated in pregnant women. Pregnancy should be avoided for at least four weeks after vaccination.[33] The vaccine’s safety for the fetus has not yet been demonstrated, although no congenital defects have been described following inadvertent administration to pregnant women.
A pregnant woman in the household is not a contraindication for immunisation of a child, and the vaccine can be administered to non-immune mothers who are breastfeeding.
24.6. Contraindications and precautions
See section 2.1.3 for pre-vaccination screening guidelines and section 2.1.4 for general contraindications for all vaccines.
24.6.1. Contraindications
24.6.1. Contraindications
Varicella vaccines are contraindicated for the following people:
- individuals with primary or acquired T-cell immune deficiency states – consult the child’s paediatrician for advice[33]
- individuals with blood dyscrasias, leukaemia, lymphomas of any type, or other malignant neoplasms affecting the bone marrow or lymphatic systems.
- individuals on high-dose steroids for more than two weeks (ie, children on 2 mg/kg per day or more of prednisone or its equivalent, or 20 mg per day if their weight is over 10 kg)
- individuals are receiving or who have recently received chemotherapy (consult with specialist)
- individuals with a family history of congenital or hereditary immunodeficiency, unless the immune competence of the potential vaccine recipient is demonstrated.
- individuals with a history of an anaphylactic reaction to a prior dose of VV or any component of the vaccine, including neomycin and gelatin.
- individuals with active untreated TB
- pregnant women – women should avoid pregnancy for at least four weeks after vaccination[33] (see section 24.5.4).
24.6.2. Precautions
24.6.2. Precautions
There is no need to avoid salicylates before or after receiving VV. People taking long-term salicylate therapy (aspirin) can receive varicella-containing vaccine, if needed. No association has been shown between the vaccine and Reye syndrome. Children on low-dose aspirin following cardiac surgery would be more at risk of thrombosis from stopping their aspirin[34] than from any theoretical risk of Reye syndrome with VV.
If tuberculin testing has to be done, it should be carried out before or simultaneously with vaccination because it has been reported that live viral vaccines may cause a temporary depression (anergy) of tuberculin skin sensitivity.[35] As this anergy may last up to a maximum of six weeks, tuberculin testing should not be performed within that period after vaccination to avoid false negative results.
On the advice of their specialist, VV may be administered to:
- patients at least two years after bone marrow transplantation
- patients at least six months after completion of chemotherapy
- HIV-positive patients who are non-immune to varicella, with mild or moderate immunosuppression.
For suggested intervals between receipt of human normal immunoglobulin or other blood products and VV, see Table A6.1 in Appendix 6.
Antiviral medications with anti-VZV activity, such as acyclovir, valaciclovir and famciclovir, can interfere with the replication of the VZV strain in VV. It is recommended that antiviral medication be stopped at least 24 hours before vaccination and recommenced at least 14 days after vaccination.
24.7. Potential responses and AEFIs
24.7.1. Potential responses
24.7.1. Potential responses
A 2013 systematic review of varicella vaccines found that mild adverse events were the most frequently reported AEFIs.[36] This includes injection-site reactions such as pain, swelling and redness, which occurred in up to 28 percent of recipients. There was no increased risk of cerebellar ataxia, encephalitis or ischaemic stroke following vaccination. Post-marketing surveillance in the US found the rate of AEFIs to be 30 per 100,000 doses of VV, and the rate of serious AEFIs was less than 4 per 100,000 doses. Fever has been reported in 15 percent of healthy children following VV and 10 percent of adults.[33, 37]
Post-VV rash
In approximately 1–3 percent of immunised children, a localised rash develops, and in an additional 3–5 percent a generalised varicella-like rash develops.[33] These rashes typically consist of two to five lesions and may be maculopapular rather than vesicular; lesions usually appear 5–26 days after immunisation. Not all rashes can be attributable to the vaccine;[33] some may be due to exposure to wild-type virus, prior to vaccination.
Transmission of vaccine virus to contacts of vaccinated individuals
In healthy vaccine recipients, transmission of vaccine virus to contacts is exceedingly rare, documented in nine immunised people and resulting in 11 secondary cases. The documented risk exists only if the immunised person develops a rash.[33] Err on the side of caution and isolate the vaccine recipient if they are a household contact of an immunocompromised individual and a post-immunisation rash occurs. If an immunocompromised individual inadvertently comes in contact with a vaccine recipient who has a varicella-like rash, the administration of zoster immunoglobulin (ZIG) and/or acyclovir should be considered (see below).[33] Intravenous acyclovir may be required if symptoms develop.
24.7.2. AEFIs
24.7.2. AEFIs
Vaccine virus shingles
The Oka strain of VZV used in the available vaccines can establish latent ganglionic infection in vaccine recipients and later reactivate to produce clinical zoster (shingles). The risk of zoster is lower, and the clinical severity milder, in immunocompentent vaccine recipients than in naturally infected children. A cohort study in children with acute lymphoblastic leukaemia (who have a high rate of zoster in childhood) showed that vaccine recipients had less than one-fifth the zoster rate of their naturally infected counterparts.[30, 38] Some zoster lesions in vaccine recipients have been shown to contain wild-type virus, likely acquired prior to vaccination.[33]
Febrile seizures with MMRV vaccine
MMRV vaccines are not currently available in New Zealand. Compared with the use of MMR and VV at the same visit, use of MMRV vaccine requires one fewer injection but is associated with a higher risk of fever and febrile seizures 5 to 12 days after the first dose among children aged 12–23 months (approximately one extra febrile seizure for every 2,300–2,600 MMRV vaccine doses).[39]
After the second dose, there are no differences in incidence of fever, rash or febrile seizures among recipients of MMRV vaccine compared with recipients of MMR and VV.[39] There is no evidence of an association with increased febrile seizures when MMRV is given to toddlers as a second dose of MMR.[40] For example, in Australia, the first dose of MMR is given at 12 months and the second dose is given as MMRV at 18 months.
24.8. Control measures
At present, VZV is not a notifiable disease in New Zealand.
24.8.1. Susceptibility
24.8.1. Susceptibility
In general, a positive history of chickenpox can be taken as indicating immunity, provided there has not been an intervening bone marrow transplant or other immunosuppressive therapy. Recall of varicella or characteristic rash is reliable evidence of immunity. In people with no history or recall of the rash, 70–90 percent are found to be immune.[33] Consult with the local laboratory about the availability and interpretation of varicella serology.
24.8.2. Post-exposure prophylaxis with zoster immunoglobulin
24.8.2. Post-exposure prophylaxis with zoster immunoglobulin
Zoster immunoglobulin (ZIG) is a high-titre immunoglobulin available from the New Zealand Blood Service for passive immunisation of varicella in high-risk individuals. It is most effective if given within 96 hours after exposure, but may be have some efficacy if given up to 10 days post-exposure.[39, 41, 42, 43] ZIG should be given intramuscularly.[43] Intravenous immunoglobulin (IVIG) can be given when ZIG is unavailable.
For further information, see Starship Child Health guidelines.
The decision whether to offer ZIG depends on:[43]
- the likelihood that the exposed person is susceptible to varicella
- the probability that a given exposure to varicella will result in infection
- the likelihood that complications would develop if the person exposed is infected.
Contact (exposure) can be classified as follows:[43]
- household contact – infection is very likely to occur in a susceptible individual living with an infected contact
- playmate contact – more than one hour of play indoors with infected individual
- newborn infant contact – when the mother of a newborn infant develops chickenpox (but not shingles) from seven days before to seven days after delivery
- hospital contact – individuals in the same two-bed room or have face-to-face contact for longer than five minutes.
Provided exposure has occurred and susceptibility is likely, ZIG is recommended for:
- pregnant non-immune women (see section 24.8.6 below and discuss with an infectious diseases physician)
- newborn infants whose mother had onset of chickenpox (but not shingles) within seven days before or after delivery (see section 24.8.6)
- hospitalised premature infants whose mothers have no history of chickenpox, or who were born at less than 28 weeks’ gestation, or with birthweight less than 1,000 g, irrespective of maternal history
- immunocompromised individuals – discuss the use of ZIG with their specialists, as appropriate.
Dosage of ZIG
ZIG prepared by CSL Behring in Melbourne, derived from human plasma donated in New Zealand, is available in single vials containing 200 IU varicella-zoster antibody. The actual volume in the vial is stated on the label. The recommended dose is based on body weight and is shown in Table 24.2 below. ZIG should be given intramuscularly, not intravenously.[43]
Table 24.2: Dose of ZIG based on body weight
|
Weight of patient (kg) |
Dose (IU) |
Number of vials |
|---|---|---|
|
0–10 |
125 |
1 |
|
10.1–20 |
250 |
2 |
|
20.1–30 |
375 |
2 |
|
30.1–40 |
500 |
3 |
|
over 40 |
600 |
3 |
|
Source: CSL Behring. 2022. Zoster Immunoglobulin-VF New Zealand Data Sheet (accessed 17 September 2024). |
||
If ZIG is not available, IVIG can be used. The titre of anti-varicella antibody will vary between lots, and the blood transfusion centre haematologist needs to be contacted to confirm the appropriate dose when IVIG is used. Refer to the immunoglobulin preparations section in the Transfusion Medicine Handbook.
24.8.3. Post-exposure vaccination and outbreak control
24.8.3. Post-exposure vaccination and outbreak control
VV may be used for post-exposure prophylaxis of susceptible individuals aged 9 months or older, if there are no contraindications to vaccine use[33] – see Table 24.3. Data from the US and Japan from household, hospital and community settings indicates that VV is effective in preventing illness or modifying varicella severity if used within three days, and possibly up to five days, of exposure.
Table 24.3: Post-exposure varicella vaccination recommendations
| Note: Funded individuals are shown in the shaded rows below. See the Pharmaceutical Schedule for any changes to the funding decisions. |
|
|
Schedule |
|---|---|
|
Immune-competent hospital in-patients who are susceptible to varicella, from age 9 monthsa |
First dose within 3 days of exposure (up to a maximum of 5 days) Second dose at least 6 weeks later |
|
Susceptible individuals aged from 9 months – who are not eligible for age-appropriate funded vaccinea,b,c |
Give 1 dose within 3 days of exposure (up to a maximum of 5 days) A second dose can be given at least 6 weeks later |
|
a. The funded 15-month VV dose can be given from 12 months of age for post-exposure prophylaxis b. VV can be purchased for individuals who are not eligible to received funded VV, including infants aged 9 months to under 12 months who do not have an eligible condition listed in Table 24.1. c. Children who were under age 12 months when they received VV for post-exposure prophylaxis will still be eligible for the age 15-month dose. Ensure there are at least 6 weeks between doses. |
|
VV may not prevent disease in all cases because some individuals may have been exposed to the same source as the index case.[33] If exposure to varicella does not result in infection, post-exposure vaccination should induce protection against subsequent exposure. If the exposure results in infection, no evidence indicates that administration of VV during the pre-symptomatic or prodromal stage of illness increases the risk for AEFIs. Note that although this method of immunisation may be successful, it is not necessarily reliable. Immunisation before exposure is therefore recommended as the preferred method of preventing outbreaks.
24.8.4. In-hospital exposure
24.8.4. In-hospital exposure
In the event of an exposure:
- susceptible staff should be excluded from contact with high-risk patients from day 8 to day 21 after exposure to varicella (or shingles in an immunocompromised patient)
- hospital staff who have no history of chickenpox and who will be in contact with pregnant women or high-risk patients should be tested for varicella zoster antibodies; vaccination is recommended for those who are not immune or whose serostatus cannot be promptly determined.
Two doses of VV are funded for post-exposure prophylaxis of immune-competent in-patients who are susceptible to varicella (see section 24.8.3).
24.8.5. Exclusion from school or early childhood education services
24.8.5. Exclusion from school or early childhood education services
Parents/guardians should be advised that:
- infected children should be excluded from early childhood education services or school until fully recovered, or all lesions have crusted. Lesions from mild breakthrough disease in immunised children may not crust but these children should be excluded until no new lesions appear for 24 hours[33]
- immune-deficient children should be excluded from early childhood education services or school until three weeks after the last documented case.
24.8.6. Care of pregnant women after exposure
24.8.6. Care of pregnant women after exposure
Pregnant women are at higher risk of severe complications from varicella. If an immune-competent pregnant woman with no history of varicella or vaccination is exposed to varicella, it is recommended, where possible, that her varicella antibodies be assessed (Figure 24.1). If there is no evidence of immunity, two possible courses of action are available: either administer ZIG, or await the onset of symptoms and as soon as possible commence the administration of acyclovir, which is effective in this situation and now regarded as safe in pregnancy. Discuss the clinical circumstances with an infectious diseases physician before deciding on which course of action is best.
Intravenous acyclovir is recommended for the pregnant woman with severe complications of varicella. ZIG given to a pregnant woman within five days of delivery may not protect the fetus/neonate: the neonate should receive ZIG on delivery and may need treatment with acyclovir (Figure 24.2).
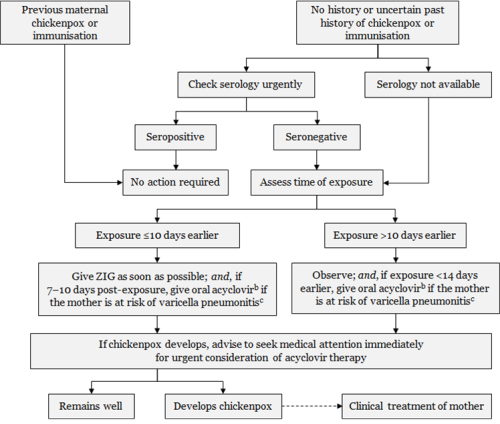
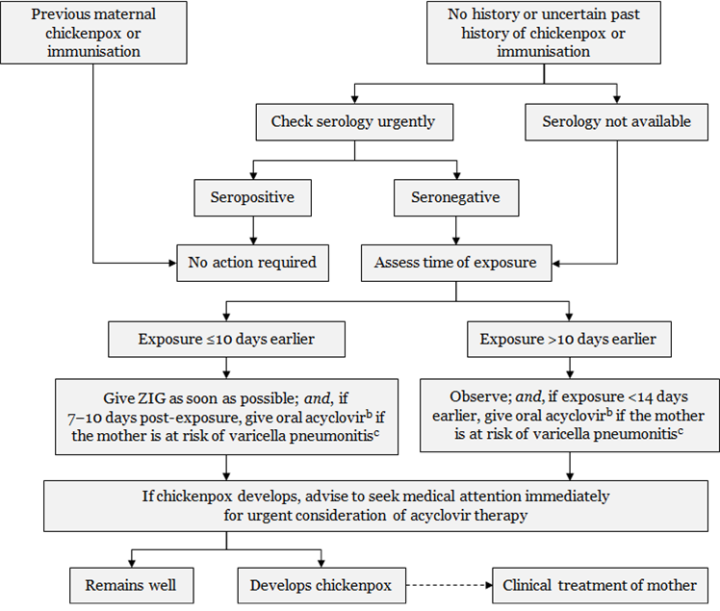
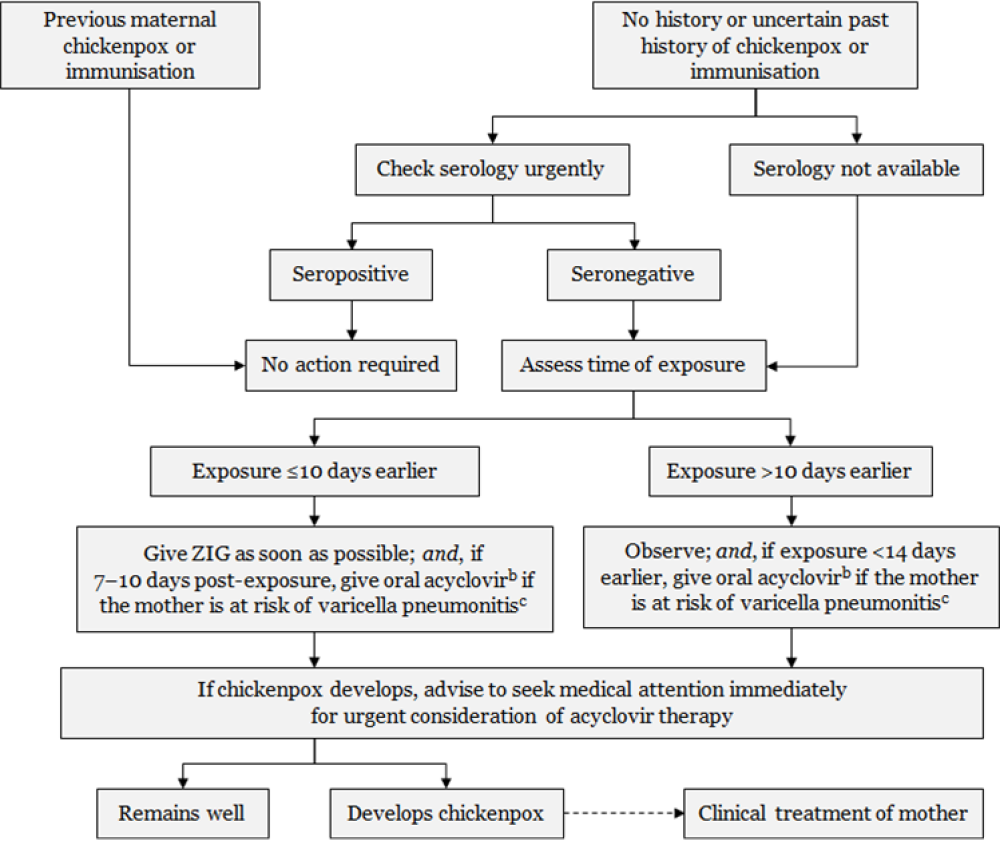
Figure 24.1: Management of pregnant women exposed to varicella or zoster
|
Every effort should be made to confirm the diagnosis in the suspected positive contact and assess significance of exposure.a Exposure or symptoms in the final two weeks of pregnancy should always be discussed with a specialist.
a. Exposure to varicella or zoster for which ZIG is indicated for susceptible persons includes: living in the same household as a person with active chickenpox or herpes zoster; face-to-face contact with a case of chickenpox for at least 5 minutes; close contact (eg, touching, hugging) with a person with active zoster. b. Efficacy of acyclovir for post-exposure prophylaxis has not been tested in controlled trials. Dose is 800 mg orally, 5 times per day for 7 days. c. The mother is at risk of pneumonitis if she is in the second half of pregnancy; has underlying lung disease; is immunocompromised; or is a smoker. Adapted from: Australasian Society for Infectious Diseases. 2014. Varicella zoster virus. In: Palasanthiran P, Starr M, Jones C, et al (eds) Management of Perinatal Infections. Sydney: Australasian Society for Infectious Diseases. |
Pregnant women exposed to VZV should be counselled about the risks of congenital varicella syndrome (CVS), a rare but devastating disorder that can occur following varicella zoster infection during pregnancy (see Table 24.4). The risk of CVS is greatest in the first 20 weeks of pregnancy. Large case studies suggest that the rate of CVS is 0.4 percent when maternal infection occurs up to week 12 of pregnancy, and 2 percent from weeks 13 to 20.
There is no single diagnostic test available for CVS. Regular fetal ultrasound for developmental anomalies is recommended. VZV fetal serology is unhelpful but amniocentesis may be considered; negative VZV PCR may be reassuring.
Table 24.4: Sequelae of congenital varicella
|
Sequelae |
Frequency |
|---|---|
|
Skin scars |
78% |
|
Eye abnormalities |
60% |
|
Limb abnormalities |
68% |
|
Prematurity, low birthweight |
50% |
|
Cortical atrophy, severe developmental delay |
46% |
|
Poor sphincter control |
32% |
|
Early death |
29% |
|
Adapted from: Australasian Society for Infectious Diseases. 2014. Varicella zoster virus. In: Palasanthiran P, Starr M, Jones C, et al (eds) Management of Perinatal Infections. Sydney: Australasian Society for Infectious Diseases. |
|
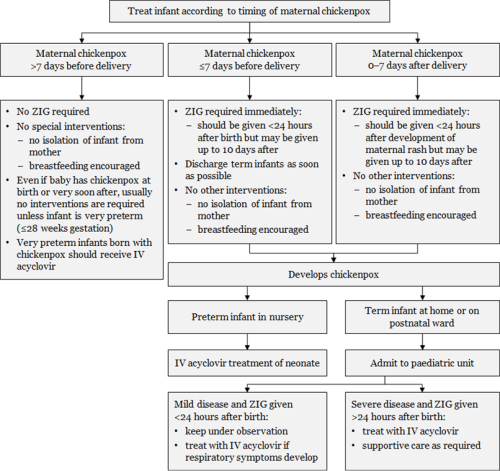
Figure 24.2: Management of infants from mothers with perinatal varicella or zoster
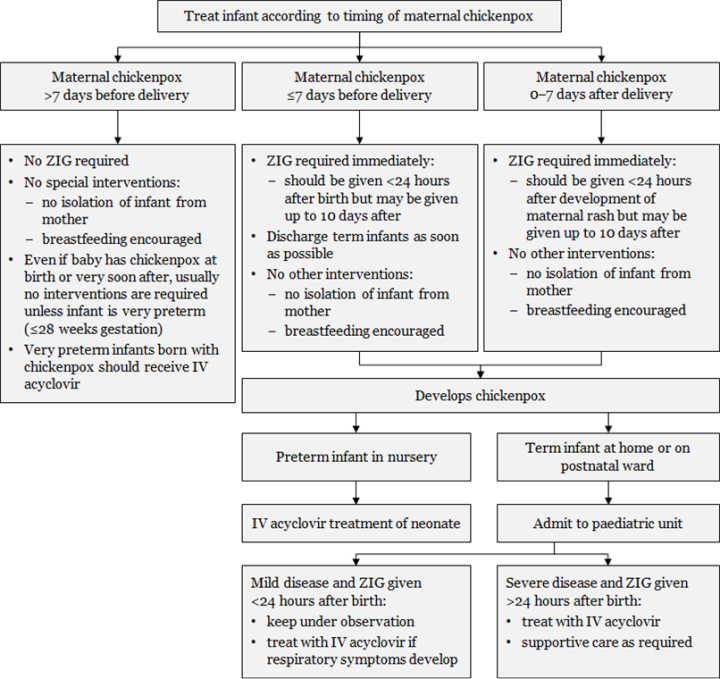
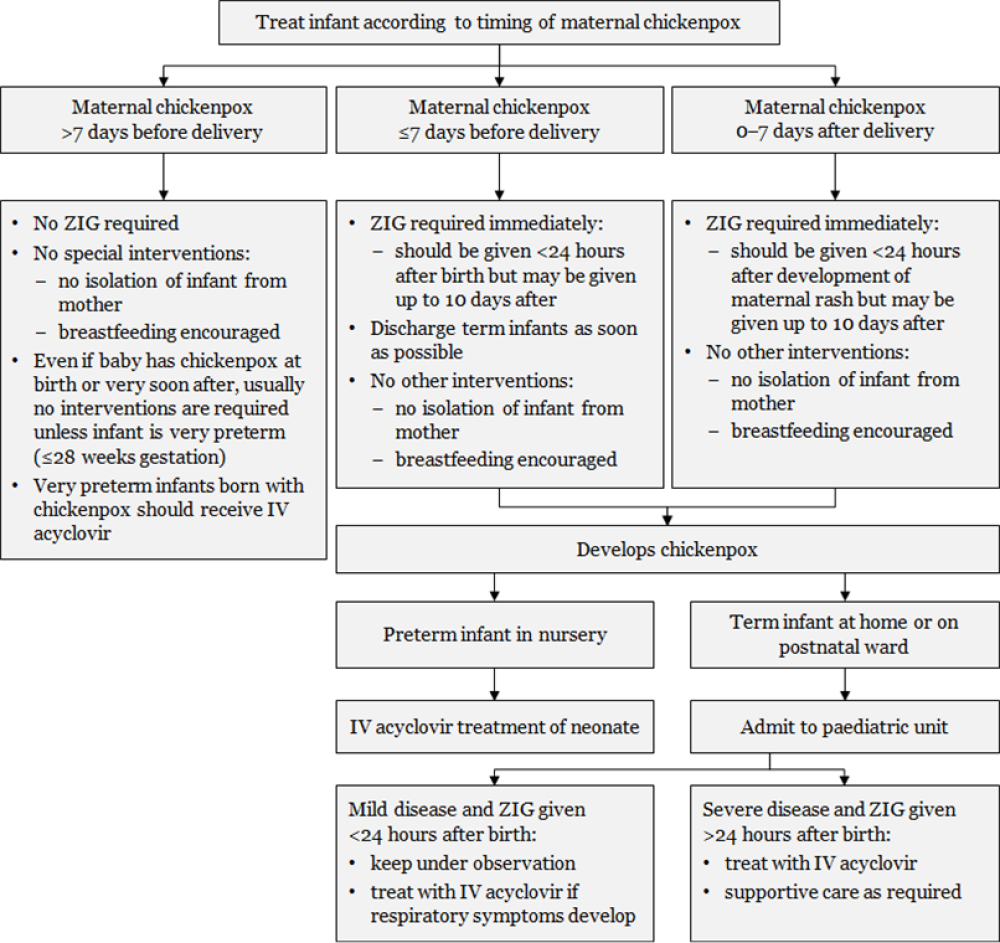
|
Notes: a. Transplacentally acquired VZV is high risk and severity is reduced by ZIG. b. ZIG is not always effective in preventing severe disease.
Adapted from: Australasian Society for Infectious Diseases. 2014. Varicella zoster virus. In: Palasanthiran P, Starr M, Jones C, et al (eds) Management of Perinatal Infections. Sydney: Australasian Society for Infectious Diseases. |
24.9. Variations from the vaccine data sheet
The VV (Varilrix) data sheet recommends that children aged 9 months to 12 years receive a second dose administered at least six weeks after the first to ensure optimal protection against varicella.[29] Health New Zealand | Te Whatu Ora instead recommends a single dose of VV for healthy children at 15 months or for catch-up at 11 years of age (see section 24.5.1) and two doses for individuals with a special groups condition given 6 weeks apart (see section 24.5.2).
The datasheet that Varilrix is to be administered subcutaneously. Health New Zealand | Te Whatu Ora recommends that parenteral live vaccines on the Schedule (MMR and varicella) be administered via intramuscular (IM) route, unless the patient is on an anticoagulant or has a bleeding disorder, in which case the preferred route is subcutaneous (SC). There are no immunogenicity concerns when varicella vaccine is given either SC or IM (see section 2.2.3).
Due to an association with Reye syndrome, salicylates and varicella infection, the vaccine manufacturers advised against the use of salicylates for six weeks after VV is given. No association has been seen with Reye syndrome, salicylates and the use of varicella vaccine. Health New Zealand recommends that non-immune individuals receiving long term salicylate therapy can receive VV, if not otherwise contraindicated, as the benefit of the vaccine likely outweighs any theoretical risk.
References
References
References
- World Health Organization. 2014 Background paper on varicella vaccine. SAGE Working Group on Varicella and Herpes Zoster Vaccines. WHO: Geneva. URL: https://terrance.who.int/mediacentre/data/sage/SAGE_Docs_Ppt_Apr2014/6_session_varicella_herpes_zoster/Apr2014_session6_varicella.pdf. (accessed 16 May 2022)
- Sengupta N ,Breuer J. A global perspective of the epidemiology and burden of varicella-zoster virus. Current Pediatric Reviews, 2009. 5(4): p. 207-228.
- Lopez AS, Zhang J, Brown C,Bialek S. Varicella-related hospitalizations in the United States, 2000–2006: the 1-dose varicella vaccination era. Pediatrics, 2011. 127(2): p. 238-45.
- Shah SS, Wood SM, Luan X,Ratner AJ. Decline in varicella-related ambulatory visits and hospitalizations in the United States since routine immunization against varicella. Pediatric Infectious Disease Journal, 2010. 29(3): p. 199-204.
- Khandaker G, Marshall H, Peadon E, et al. Congenital and neonatal varicella: impact of the national varicella vaccination programme in Australia. Archives of Disease in Childhood, 2011. 96(5): p. 453-6.
- Carville KS, Riddell MA ,Kelly HA. A decline in varicella but an uncertain impact on zoster following varicella vaccination in Victoria, Australia. Vaccine, 2010. 28(13): p. 2532-8.
- Leung J, Harpaz R, Molinari NA, et al. Herpes zoster incidence among insured persons in the United States, 1993–2006: evaluation of impact of varicella vaccination. Clinical Infectious Diseases, 2011. 52(3): p. 332-40.
- Reynolds MA, Chaves SS, Harpaz R, et al. The impact of the varicella vaccination program on herpes zoster epidemiology in the United States: a review. Journal of Infectious Diseases, 2008. 197 Suppl 2(Suppl 2): p. S224-7.
- Hales CM, Harpaz R, Joesoef MR,Bialek SR. Examination of links between herpes zoster incidence and childhood varicella vaccination. Annals of Internal Medicine, 2013. 159(11): p. 739-45.
- Wen SC, Best E, Walls T, et al. Prospective surveillance of hospitalisations associated with varicella in New Zealand children. Journal of Paediatrics and Child Health, 2015. 51(11): p. 1078-83.
- Wen SC, Miles F, McSharry B,Wilson E. Varicella in a paediatric intensive care unit: 10-year review from Starship Children's Hospital, New Zealand. Journal of Paediatrics and Child Health, 2014. 50(4): p. 280-5.
- Ministry of Health. Hospital event data and stats. [updated 28 October 2021]; URL: https://www.health.govt.nz/nz-health-statistics/health-statistics-and-data-sets/hospital-event-data-and-stats. (accessed 10 May 2022)
- Ministry of Health. Mortality data and stats. [updated 16 December 2021]; URL: https://www.health.govt.nz/nz-health-statistics/health-statistics-and-data-sets/mortality-data-and-stats. (accessed 10 May 2021)
- Chang LY, Huang LM, Chang IS,Tsai FY. Epidemiological characteristics of varicella from 2000 to 2008 and the impact of nationwide immunization in Taiwan. BMC Infectious Diseases, 2011. 11(16 Dec): p. 352.
- Pozza F, Piovesan C, Russo F, et al. Impact of universal vaccination on the epidemiology of varicella in Veneto, Italy. Vaccine, 2011. 29(51): p. 9480-7.
- Siedler A ,Arndt U. Impact of the routine varicella vaccination programme on varicella epidemiology in Germany. Euro Surveillance, 2010. 15(13): p. pii=19530.
- Tan B, Bettinger J, McConnell A, et al. The effect of funded varicella immunization programs on varicella-related hospitalizations in IMPACT centers, Canada, 2000-2008. Pediatric Infectious Disease Journal, 2012. 31(9): p. 956-63.
- World Health Organization. 2014 Systematic review of available evidence on effectiveness and duration of protection of varicella vaccines. SAGE Working Group on Varicella and Herpes Zoster Vaccines. Geneva. URL: http://www.who.int/immunization/sage/meetings/2014/april/presentations_background_docs/en/. (accessed 3 July 2020)
- Marin M, Guris D, Chaves SS, et al. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR: Recommendations and Reports, 2007. 56(RR-4): p. 1-40.
- Quinn HE, Gidding HF, Marshall HS, et al. Varicella vaccine effectiveness over 10 years in Australia; moderate protection from 1-dose program. Journal of Infection, 2019. 78(3): p. 220-225.
- American Academy of Pediatrics Committee on Infectious Diseases. Prevention of varicella: recommendations for use of varicella vaccines in children, including a recommendation for a routine 2-dose varicella immunization schedule. Pediatrics, 2007. 120(1): p. 221-31.
- Marin M, Meissner HC ,Seward JF. Varicella prevention in the United States: a review of successes and challenges. Pediatrics, 2008. 122(3): p. e744-51.
- Chan YD, Edmunds WJ, Chan HL, et al. Varicella vaccine dose depended effectiveness and waning among preschool children in Hong Kong. Human Vaccines & Immunotherapeutics, 2019: p. 1-7.
- Gershon A, Marin M ,Seward JF. 2018. Varicella vaccines, in Plotkin's Vaccines (7th edition), Plotkin S, Orenstein W, Offit P, and Edwards K (eds). Elsevier: Philadelphia, US.
- Halperin SA, Ferrera G, Scheifele D, et al. Safety and immunogenicity of a measles-mumps-rubella-varicella vaccine given as a second dose in children up to six years of age. Vaccine, 2009. 27(20): p. 2701-6.
- Leung J, Bialek SR ,Marin M. Trends in varicella mortality in the United States: Data from vital statistics and the national surveillance system. Human Vaccines & Immunotherapeutics, 2015. 11(3): p. 662-8.
- Waye A, Jacobs P ,Tan B. The impact of the universal infant varicella immunization strategy on Canadian varicella-related hospitalization rates. Vaccine, 2013. 31(42): p. 4744-8.
- Streng A, Grote V, Carr D, et al. Varicella routine vaccination and the effects on varicella epidemiology - results from the Bavarian Varicella Surveillance Project (BaVariPro), 2006-2011. BMC Infectious Diseases, 2013. 13: p. 303.
- GSK, Varilrix New Zealand Data Sheet. 2019.
- LaRussa P, Steinberg S ,Gershon AA. Varicella vaccine for immunocompromised children: results of collaborative studies in the United States and Canada. Journal of Infectious Diseases, 1996. 174 Suppl 3(Suppl 3): p. S320-3.
- Son M, Shapiro ED, LaRussa P, et al. Effectiveness of varicella vaccine in children infected with HIV. Journal of Infectious Diseases, 2010. 201(12): p. 1806-10.
- Holmes CN, Iglar KT, McDowell BJ,Glazier RH. Predictive value of a self-reported history of varicella infection in determining immunity in adults. CMAJ: Canadian Medical Association Journal, 2004. 171(10): p. 1195-6.
- American Academy of Pediatrics. 2018. Varicella-zoster infections. in Red Book: 2018 Report of the Committee on Infectious Diseases, Committee on Infectious Diseases, Kimberlin D, Brady M, et al. (eds). URL: https://redbook.solutions.aap.org/redbook.aspx. (accessed 3 July 2020)
- Li JS, Yow E, Berezny KY, et al. Clinical outcomes of palliative surgery including a systemic-to-pulmonary artery shunt in infants with cyanotic congenital heart disease: does aspirin make a difference? Circulation, 2007. 116(3): p. 293-7.
- Brickman HF, Beaudry PH ,Marks MI. The timing of tuberculin tests in relation to immunization with live viral vaccines. Pediatrics, 1975. 55(3): p. 392-6.
- World Health Organization. 2013 Safety of varicella and MMRV vaccines: a systematic review. SAGE Working Group on Varicella and Herpes Zoster Vaccines. WHO: Geneva. URL: https://terrance.who.int/mediacentre/data/sage/SAGE_Docs_Ppt_Apr2014/6_session_varicella_herpes_zoster/Apr2014_session6_varicella_MMRV_safety.pdf. (accessed 16 May 2022)
- Australian Technical Advisory Group on Immunisation. 2018. Australian Immunisation Handbook (ed.), Canberra: Australian Government Department of Health. URL: https://immunisationhandbook.health.gov.au/ (accessed October 2019)
- Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005-2009. Journal of Infectious Diseases, 2013. 208(11): p. 1859-68.
- Marin M, Broder KR, Temte JL, et al. Use of combination measles, mumps, rubella, and varicella vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR: Recommendations and Reports, 2010. 59(RR-3): p. 1-12.
- Macartney K, Gidding HF, Trinh L, et al. Evaluation of combination measles-mumps-rubella-varicella vaccine introduction in Australia. JAMA Pediatr, 2017. 171(10): p. 992-998.
- Centers for Disease Control and Prevention. FDA approval of an extended period for administering VariZIG for postexposure prophylaxis of varicella. Morbidity and Mortality Weekly Report, 2012. 61(12): p. 212.
- Centers for Disease Control and Prevention. Updated recommendations for use of VariZIG--United States, 2013. MMWR: Morbidity and Mortality Weekly Report, 2013. 62(28): p. 574-6.
- Starship Child Health. Zoster Immunoglobulin – Starship Clinical Guidelines. [updated 22 February 2017 ]; URL: https://www.starship.org.nz/guidelines/zoster-immunoglobulin/. (accessed 17 February 2020)