On this page
Key information
|
Mode of transmission |
By contact with infected nasopharyngeal secretions. Infants with congenital rubella syndrome (CRS) shed rubella virus in their pharyngeal secretions and urine. |
|---|---|
|
Incubation period |
14–23 days, usually 16–18 days. Up to 50 percent of rubella infections are subclinical. |
|
Period of communicability |
7 days before until 7 days after the onset of the rash. Infants with CRS may be infectious for months. |
|
Incidence and burden of disease |
Endemic rubella was verified as eliminated in 2017. One imported case of rubella was notified in 2018. Cases continue in other regions, including China. |
|
Funded vaccine |
MMR is a live attenuated vaccine. |
|
Dose, presentation, route |
0.5 mL per dose after reconstitution. Pre-filled syringe and glass vial. The vaccine must be reconstituted prior to injection. Intramuscular or subcutaneous injection. |
|
Funded vaccine indications and schedule |
Children at ages 12 months and 15 months. Adults who are susceptible to one or more of measles, mumps and rubella. For (re)vaccination following immunosuppression, if the individual is immunocompetent enough to safely receive the vaccine. |
|
Recommended |
All adults born since January 1969 should be up to date with two doses of MMR or have evidence of immunity to all three vaccine components. It is particularly important for health care workers, individuals who work with children, armed forces personnel, staff of correctional facilities, long-term care facilities and immigration/refugee centres and laboratory staff. All vaccine-eligible travellers, particularly to high-risk countries. |
|
Vaccine efficacy/ effectiveness |
Highly effective, 2 doses are anticipated to provide lifelong protection. Protection is best achieved through herd immunity from high immunisation coverage. |
|
Contraindication |
MMR is contraindicated for those with anaphylaxis to neomycin, immunocompromise and in pregnancy. See section 12.6 (external link) for cautions around receipt of blood products and other live vaccines, and other precautions. |
|
Precautions and special considerations |
All pregnant women and women planning pregnancy should have their immunisation history checked. A woman is immune to rubella if she has had two documented doses of a rubella-containing vaccine given at least 4 weeks apart and given after age 12 months, regardless of serology. Pregnant non-immune women should avoid contact with known cases of rubella and should receive MMR after delivery. |
|
Potential response to vaccine |
MMR is generally well tolerated. Temporary joint pain 2–4 weeks after vaccination due to rubella component of the vaccine is more common in adults than children. See section 12.7. Alert parents of possible febrile seizure risk, particularly for those with a history of seizure. |
|
Public health measures |
All suspected cases must be notified immediately on suspicion. (see section 21.8) |
|
Post-exposure prophylaxis |
For management and vaccination of contacts, see section 21.8. |
21.1. Virology
Rubella is an enveloped RNA virus from the family Togaviridae and the genus Rubivirus. It is less stable than measles virus and can be inactivated by solvents, trypsin, formalin, extremes of heat and pH, and light.[1]
21.2. Clinical features
The purpose of the rubella immunisation programme is to prevent congenital rubella syndrome (CRS).
Rubella infection during pregnancy can result in fetal infection, causing CRS in a high proportion of cases. Rubella infection in the first 12 weeks of pregnancy results in fetal damage in up to 85 percent of infants, and multiple defects are common. The risk of damage declines to 50 percent by about 16 weeks’ gestation and 25 percent by the end of the second trimester.[2] Fetal abnormalities are rare from infection in the third trimester of pregnancy.
Infants born with CRS may have cataracts, nerve deafness, cardiac malformations, microcephaly, mental retardation and behavioural problems. Inflammatory changes may also be found in the liver, lungs and bone marrow. Some infected infants may appear normal at birth, but have nerve deafness, oesophageal and eye defects, and diabetes detected later.[3] CRS is one of the few known causes of autism.[2]
Clinical features of rubella in children and adults include a transient erythematous rash and lymphadenopathy without respiratory symptoms. Arthritis or arthralgia is relatively common and a classic feature of infection in adults. While usually a mild childhood illness, rubella may also present as a more severe illness, clinically indistinguishable from measles. Encephalitis occurs with a prevalence of approximately 1 in 6,000 cases and may result in residual neurological damage or, occasionally, death. Thrombocytopenia rarely occurs.
Clinical diagnosis is unreliable because the symptoms are often fleeting and can be mimicked by other viruses. In particular, the rash is not diagnostic of rubella. Up to 50 percent of rubella infections are subclinical or asymptomatic. Asymptomatic cases are also able to transmit the virus. A history of rubella should therefore never be accepted as proof of immunity without laboratory confirmation.[3]
Transmission of rubella is through direct or indirect contact with infected nasopharyngeal secretions and droplets. The incubation period is usually 16–18 days (range 14–23 days) and infectivity is between seven days before and seven days after the onset of the rash. Infants with congenital rubella syndrome (CRS) shed rubella virus in their pharyngeal secretions and urine for months after birth and should be considered infectious until they are aged 12 months.
Although the vaccine virus is excreted after vaccination, mostly from the pharynx, transmission to susceptible contacts has not been demonstrated (see section 12.7.2 (external link)). Therefore, a recently immunised contact is not a risk to a pregnant woman.
The frequency of complications and consequences of rubella infection are best described from the 1963/1964 US outbreak, involving 12.5 million cases of rubella and 30,000 infants damaged by intrauterine rubella, an incidence rate of 1 per 100 pregnancies (see Table 21.1 (external link)).
Table 21.1: Estimated morbidity and mortality associated with the 1963/64 US rubella epidemic
Table 21.1: Estimated morbidity and mortality associated with the 1963/64 US rubella epidemic
|
Total number of cases of rubella: |
12,500,000 |
|---|---|
|
Complications of rubella |
Risk per case |
|
Arthritis or arthralgia |
13 per 1,000 |
|
Encephalitis |
17 per 100,000 |
|
Neonatal deaths |
17 per 100,000 |
|
Complications caused by congenital rubella syndrome (CRS) |
Numbers of cases |
|
Total number with CRS |
20,000 |
|
Deaf children |
8,055 (40%) |
|
Deaf–blind children |
3,580 (18%) |
|
Mentally disabled children |
1,790 (9%) |
|
Adapted from: Reef SE, Plotkin S. 2018. Rubella vaccines. In: Plotkin S, Orenstein W, Offit P, et al (eds) Plotkin’s Vaccines (7th Edition). Philadelphia, US: Elsevier. Table 53.7. |
|
Rubella infection can occur (very rarely) in individuals with either naturally acquired or vaccine-induced antibody.[3] Rare cases of CRS have been reported after reinfection during pregnancy.[3]
As with measles, public health measures of accurately diagnosing potential cases of rubella with notification and contact tracing are critical (see section 21.8 (external link)).
21.3. Epidemiology
21.3.1. Global burden of disease
21.3.1. Global burden of disease
Humans are the only source of rubella infection. Infection is often asymptomatic. In the pre-vaccine era the highest incidence of clinical cases occurred in the spring among 5–9-year-old children, and 80–90 percent of adults were immune to rubella. Extensive outbreaks of rubella occurred every six to nine years, in which many children were affected by CRS. Immunisation against rubella, introduced to prevent the occurrence of CRS, has resulted in a very significant reduction in infection, especially once vaccination was introduced to boys and girls.
The Global Vaccine Action Plan set a specific target to eliminate measles and rubella by 2020 and to reduce the incidence of CRS from 100,000 cases per year. The number of notified rubella cases fell from over 670,000 in 2000 to just over 22,000 in 2016. However, from January to September 2019, nearly 40,000 cases were reported: 92 percent of those cases were in China and 7 percent in Japan.[4]
Although rubella elimination has been verified in 81 countries, less than 70 percent of infants across 168 countries worldwide were immunised against rubella by the end of 2018.[5 (external link)]
21.3.2. New Zealand epidemiology
21.3.2. New Zealand epidemiology
Rubella immunisation was introduced in 1970 (see Appendix 1 (external link)), and rubella has been a notifiable disease since 1996. The last large rubella outbreak in 1995–1996 mostly involved young adult males, who would not have been offered immunisation. This emphasises the need to immunise both boys and girls to reduce the risk of exposure in pregnant women, as well as to reduce illness in men.
Two cases of rubella were notified in 2019, in a male and a female both aged 30–39 years.
Rubella was verified as eliminated from New Zealand in October 2017. There have been no reported cases of CRS in New Zealand since 1998. See Figure 20.1
For further information, see the PHF Science (formerly ESR)’s notifiable disease reports (external link).
Figure 21.1: Rubella notifications and laboratory-confirmed cases by year, 1997–2019
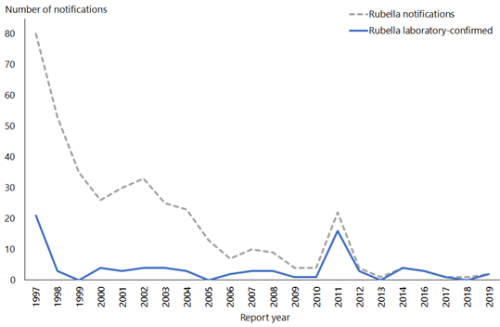
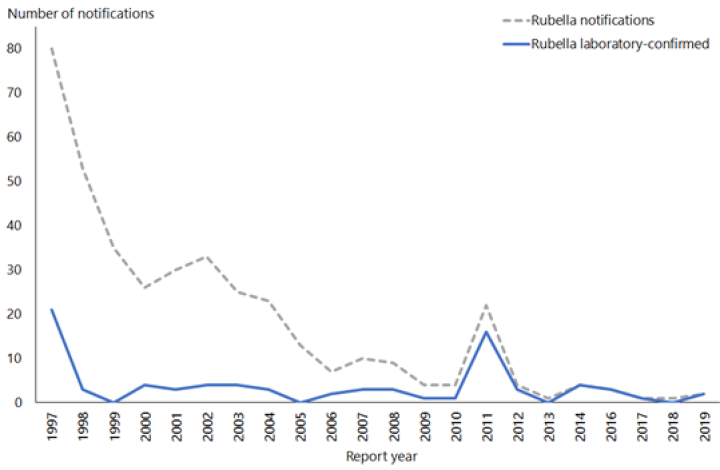
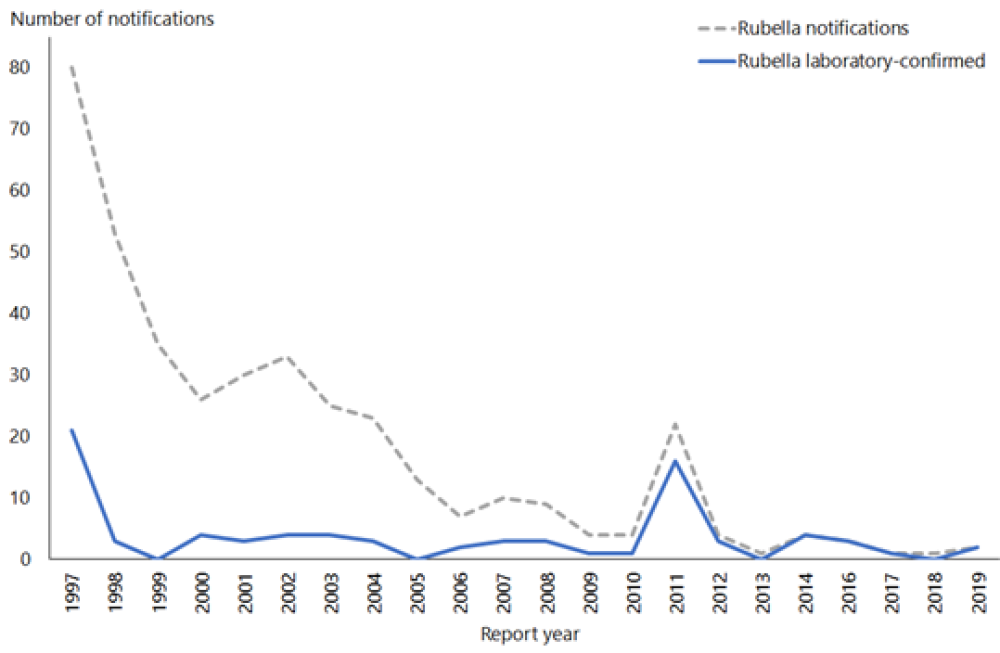
| Source: ESR |
21.4. Vaccines
21.4.1. Available vaccines
21.4.1. Available vaccines
Rubella vaccine is one of the components of the live attenuated MMR vaccine (see section 12.4.1) (and also MMRV vaccine mentioned in section 24.4.1).
Funded vaccine
MMR funded as part of the Schedule is Priorix (GSK), which contains attenuated Schwarz strain measles, RA 27/3 rubella and Jeryl Lynn mumps. (See section 12.4.1 for more information.)
Other vaccines
MMR II (MSD) was the funded vaccine prior to the 1 July 2017 Schedule change (see section 12.4.1 (external link)).
21.4.2. Efficacy and effectiveness
21.4.2. Efficacy and effectiveness
The rubella vaccine has been shown to be 90–97 percent effective in an outbreak after a single dose, and this is likely to be higher with a two‑dose schedule. One dose of rubella vaccine at 12 months or older induces an antibody response in at least 95 percent of recipients. Studies have found no evidence of waning of protection over decades of follow‑up.[3] In more than 90 percent of recipients, antibodies persisted for at least 16 years; other studies have reported persistence up to 21 years.[3] A few recipients fail to produce antibodies following immunisation, and a small number of individuals lose antibodies, whether they are derived from natural infection or the vaccine. As part of a follow-up of those who received MMR in Finland, it was shown that while antibodies wane over time, immunity to rubella remained secure 20 years after the second dose of MMR.[6 (external link)]
21.4.3. Transport, storage and handling
21.4.3. Transport, storage and handling
Transport according to the National Standards for Vaccine Storage and Transportation for Immunisation Providers 2017 (2nd edition).
Store at +2°C to +8°C. Do not freeze.
MMR must be reconstituted only with the diluents supplied by the manufacturer. Use MMR as soon as possible after reconstitution. If storage is necessary, reconstituted vaccine can be stored at +2°C to +8°C for up to eight hours.
21.4.4. Dosage and administration
21.4.4. Dosage and administration
The dose of MMR is all of the reconstituted vaccine (approximately 0.5 mL) administered by intramuscular injection, or subcutaneous injection if indicated (see section 2.2.3).
Co-administration with other vaccines
MMR can be given concurrently with other vaccines, as long as separate syringes are used and the injections are given at different sites. If not given concurrently, live vaccines should be given at least four weeks apart. (See also section 2.2.7 (external link) for information about multiple injections at the same visit.)
Interchangeability
The two brands of MMR (Priorix and MMR II) may be used interchangeably for completion of a course.[1 (external link)]
21.5. Recommended immunisation schedule
To prevent all cases of CRS, rubella must not circulate in the community. Achieving at least 95 percent coverage of two doses of MMR prevents the circulation of rubella (which is much less infectious than measles). As in other high-income countries, New Zealand’s primary strategy for preventing and maintaining elimination of rubella is to vaccinate both boys and girls (Table 21.2 (external link)).
Historically, a targeted individual protection approach was used in which only 11‑year-old girls were immunised with one dose of rubella vaccine. Even with high coverage, there remained women of childbearing age who were susceptible to rubella due to failure to be vaccinated or vaccine failure and cases of CRS continued to occur albeit at a reduced rate. Rubella remained in circulation in New Zealand because children aged under 11 years and males were not vaccinated.
Table 21.2: Recommended MMR vaccination schedule
Table 21.2: Recommended MMR vaccination schedule
|
|
Schedule |
|---|---|
|
Usual childhood schedulea |
2 doses: at ages 12 months and 15 months |
|
Catch-upb for children adolescents and adults |
2 doses: at least 4 weeks apart |
|
a. If MMR is given to children aged 4 months to under 11 months for outbreak control, 2 further MMR doses are still required at ages 12 months (at least 4 weeks after previous dose) and 15 months. b. MMR is funded for those who are susceptible to 1 or more of the 3 diseases. See sections 21.5.2 (external link) and 21.5.3 |
|
21.5.1. Usual childhood schedule
21.5.1. Usual childhood schedule
Two doses of rubella vaccine as MMR are recommended at age 12 months and 15 months. Over 95 percent of individuals will become immune to rubella after one dose.[2] The second dose is not a booster. Two doses are recommended because nearly all the 2–5 percent not protected by the first dose will be protected by the second. The second dose of vaccine can be given as soon as four weeks after the first dose. (See below for the recommendations for other groups.)
Children who receive MMR vaccination during an outbreak (of measles, mumps or rubella) when aged under 11 months (MMR0) require two further doses administered at age 12 months and 15 months. No opportunity should be missed to achieve immunity, if not contraindicated.
21.5.2. Catch-up
21.5.2. Catch-up
Any individual born on or after 1 January 1969 (see section 12.5.2) who does not have two documented doses of MMR, given at least four weeks apart with the first dose given any time after age 12 months, should be offered either one or two doses (four weeks apart) to bring them up to two doses (funded).
Even if the individual has previously received single-antigen measles vaccine, up to two doses of MMR (ie, additional doses of measles vaccine) may be given to these individuals to ensure rubella and mumps protection. There are no significant adverse effects from further vaccinating individuals who are already immune to measles, mumps and/or rubella, and no reliance can be placed on a prior clinical history of rubella infection.
Immigrants to New Zealand
The vaccination status of immigrants should be checked as a priority group: particularly, those born overseas (especially in Asia, the Pacific Islands, sub-Saharan Africa and South America) who entered New Zealand after the age of routine vaccination.
Anyone who does not have two documented doses of MMR, given any time after age 11 months, should be offered either one or two doses (four weeks apart) to bring them up to two doses (funded if eligible). Some countries provide a rubella-containing vaccine before the age of 11 months; these children may require extra MMR doses to be fully immunised.
Occupational risk
Visitors to countries with circulating rubella virus, including those travelling for work or humanitarian purposes, are at highest risk of importing disease. It is recommended to be fully immunised prior to departure from New Zealand. During an outbreak, health care workers and those working with infants in day care facilities are at high risk of transmitting disease to pregnant women and their infants. MMR is funded for all New Zealand residents born since 1 January 1969 if they have not previously had two documented doses of MMR.
21.5.3. Pregnancy and breastfeeding
21.5.3. Pregnancy and breastfeeding
Women planning pregnancy
It is particularly important to ensure that women of child-bearing age are immune to rubella.[1] Women who are planning pregnancy should have their immunisation history checked for having received two documented doses of a rubella-containing vaccine given at least four weeks apart and given after age 12 months. Non-immune women may receive MMR before pregnancy, but pregnancy should be avoided for four weeks after the last MMR vaccination.[7, 8]
As seen with recent measles and mumps outbreaks, community immunity in the 15–29 years age group is low due to historically poor MMR vaccination coverage during the 1990s and early 2000s; there is a cohort of women of child-bearing age potentially susceptible to rubella.
Pregnant women
MMR is contraindicated during pregnancy.
All pregnant women should have their immunisation history checked. A pregnant woman is considered immune to rubella if she has had two documented doses of a rubella-containing vaccine given at least four weeks apart and given after age 12 months, regardless of serology. As soon as possible following delivery, as appropriate, give one or two doses of MMR four weeks apart to women who are not immune (funded).
Serological testing for immunity to rubella is not usually performed in New Zealand except as part of routine antenatal care. Improved documentation and effective surveillance showing the rarity of CRS when there is high immunisation coverage has led to some countries, such as England, discontinuing routine antenatal rubella screening.[9] Also, the screening tests used for rubella serology can potentially give inaccurate results and may cause unnecessary stress for women.[9]
In general, it should be remembered that the great majority of New Zealand-born individuals who received all scheduled childhood vaccines will be immune to rubella. Although the chance of being exposed in New Zealand to an infectious case is becoming increasingly rare, rubella remains endemic in some countries, and the risk remains to those who are unimmunised when travelling overseas or through an imported case. (If exposure during pregnancy does occur, see the guidelines in section 21.8.2.)
After delivery
If MMR and Rhesus anti-D IG are required after delivery, both the vaccine and anti-D IG may be given at the same time, in separate sites with separate syringes. The vaccine may be given at any time after the delivery. Anti-D IG does not interfere with the antibody response to the vaccine, but whole blood transfusion does inhibit the response in up to 50 percent of vaccine recipients (see section A6.4.1).
Breastfeeding
There is no risk to the mother or child in giving MMR to breastfeeding women.[3 (external link)]
21.5.4. Immunocompromise
21.5.4. Immunocompromise
MMR is contraindicated in immunocompromised children (see section 4.2.5 (external link)). They can be partially protected from exposure to infection by ensuring that all contacts are fully immunised, including hospital staff and family members. There is no risk of transmission of MMR vaccine viruses from a vaccine recipient to the immunocompromised individual. See section 12.7.2 (external link).
MMR is funded for (re)vaccination following immunosuppression. However, it is important to be sure that the individual is immunocompetent enough to safely receive the vaccine.
HIV infection
Discuss vaccination of individuals with HIV infection with their specialists (see ‘HIV infection’ in section 4.3.12).
MMR is recommended for all HIV-positive children, whether symptomatic or asymptomatic, if their CD4+ lymphocyte percentage is 15 percent or greater. It is recommended that asymptomatic children who are not severely immunocompromised receive MMR from age 12 months to provide early protection against the three diseases. Susceptible HIV-positive children and adults aged 14 years and older may receive MMR if the CD4+ lymphocyte count is 200 cells/mm3 or greater. Administration of MMR with CD4+ counts below these recommended levels has been associated with vaccine-related pneumonitis (from the measles component).[7]
21.6. Contraindications and precautions
See also section 2.1.3 for pre-vaccination screening guidelines and section 2.1.4 (external link) for general vaccine contraindications.
21.6.1. Contraindications
21.6.1. Contraindications
See section 12.6.1 (external link) for specific MMR contraindications.
The general contraindications that apply to all immunisations are relevant to MMR.
Anaphylaxis to a previous dose of MMR or any of the vaccine components (including neomycin) is a contraindication to a further dose of MMR.
MMR should not be given to women who are pregnant, and pregnancy should be avoided for four weeks after immunisation.[2, 3] However, inadvertent immunisation with a rubella-containing vaccine in early pregnancy is no longer considered an indication for termination of pregnancy. There have been no cases of teratogenic damage from vaccine virus despite intensive surveillance in the US, the UK and Germany.[3 (external link)]
21.6.2. Precautions
21.6.2. Precautions
Egg allergy, including anaphylaxis, is not a contraindication for MMR. See section 12.6.3 for more information, and section 12.6.2 (external link) for further precautions.
For infants aged under 12 months, please discuss immunomodulatory therapies taken during pregnancy with infant’s mother or specialist, or contact IMAC before administration of MMR0 in an outbreak situation. See section 4.2.5.
21.7. Potential responses and AEFIs
See also section 12.7.
21.7.1. Potential responses
21.7.1. Potential responses
A fever of 39.4°C or more occurs in 5–15 percent of children 6 to 12 days after immunisation and generally lasts 1 to 2 days.[7] A rash may also occur in approximately 5 percent of children at the same interval post-vaccination: these children are not infectious to others, unless the rash is caused by a different concurrent illness.[7] The majority of these events are coincidental and not caused by the vaccine.[10] Serological tests or PCR can be expected to be positive if performed during this time, so testing should not be routinely performed.
Joint symptoms may be reported in 0.5 percent of young children and 10–25 percent of post-pubertal women.[2] Symptoms begin one to three weeks after immunisation and are usually transient. The prevalence of joint symptoms following rubella immunisation is lower than occurs with natural infection at a corresponding age.[2]
It was previously thought that the rubella vaccine might lead to long-term arthritis. A 2012 Institute of Medicine review concluded that the evidence was inadequate to accept or reject a causal relationship between MMR and chronic arthritis in women.[11 (external link)]
21.7.2. AEFIs
21.7.2. AEFIs
ITP and, rarely, neurological disturbances have been reported (see section 12.7.2).
21.8. Public health measures
Rubella (including congenital rubella syndrome) is a notifiable disease, and suspected cases should be notified by the clinician on suspicion to the local medical officer of health. Accurate diagnosis requires laboratory confirmation.
For detailed information about disease control measures see the 'Rubella' chapter of the Communicable Disease Control Manual.
21.8.1. Management of non-pregnant contacts
21.8.1. Management of non-pregnant contacts
The local medical officer of health will advise on contact management. Check the immunisation status of all close contacts.
Rubella-containing vaccine does not provide protection if given after exposure to rubella. However, if the exposure did not result in infection, the vaccine would induce protection against subsequent infection. Human normal immunoglobulin does not prevent rubella infection after exposure and should not be used for that purpose.[12 (external link)]
21.8.2. Management of pregnant contacts
21.8.2. Management of pregnant contacts
It is critical to accurately document the rubella status of all people who may have rubella and potentially exposed a pregnant woman to the virus. Such people will have travelled overseas or had contact with an infected returned traveller. As described in section 21.3.2 (external link), rubella virus does not circulate in New Zealand. Rubella infection in the first half of pregnancy is potentially devastating, and every possible exposure of a pregnant woman should be discussed with the local medical officer of health, obstetrician and microbiologist or infectious diseases physician.
Pregnant contacts with confirmed immunity can be reassured that the likelihood of rubella infection is remote. This applies if:
- she has received at least two documented doses of rubella-containing vaccine, or
- a previous antibody screening test has detected a protective level of antibodies, and this has been documented, or
- one dose of vaccine followed by a rubella antibody screening test showing a protective level of antibodies has been documented.
Coordinated care and management
Coordinated care and management are essential (Table 21.3). An obstetrician (or a maternal fetal medicine specialist) and an infectious diseases specialist/microbiologist should be consulted when the diagnosis of possible rubella infection in a pregnant woman is first considered. The clinical picture and all test results should be discussed by all involved in the care of the woman, to enable an accurate interpretation of the serological results before advising the woman about the risk to her fetus and options regarding the continuation of pregnancy.
Pregnant women whose immunity to rubella has not been confirmed for the current pregnancy, and who have been exposed to rubella in the first half of pregnancy, must be investigated serologically irrespective of immunisation history or history of previous clinical rubella. Serum should be obtained as soon as possible, with the clinical details included on the request form. The laboratory should be asked to store an aliquot of serum for later testing in tandem with a follow-up sample. These results must be interpreted in conjunction with the time since exposure, to determine whether acute infection has occurred.
It is essential to discuss testing with the local clinical microbiologist before taking samples, to ensure that the right samples are obtained, and the best tests performed expeditiously. All requests to laboratories must state the:
- duration of pregnancy and last menstrual period
- date of exposure to possible rubella
- date of blood specimen
- name of the index case who is thought to have rubella.
The use of IG is not recommended for post-exposure prophylaxis of rubella in early pregnancy or any other circumstance. However, IG may be considered if termination of the pregnancy is not an option, but termination must be discussed when maternal infection is confirmed. Although IG has been shown to reduce clinically apparent infection in the mother, there is no guarantee that fetal infection will be prevented.
Table 21.3: Suggested roles of health professionals
|
|
Lead maternity carer |
Medical officer of health |
GP |
Obstetrician/ |
|---|---|---|---|---|
|
Check rubella status in every pregnancy (2 documented doses of rubella-containing vaccine) |
✓ |
|
|
|
|
Investigate initial suspected rubella case and trace contacts |
|
✓ |
|
|
|
Coordinate care of exposed non-immune pregnant woman |
|
✓ | ✓ |
|
|
Review clinical and laboratory results, and discuss options with the pregnant woman if rubella is confirmed |
|
|
|
✓ |
|
AFTER delivery – vaccinate any woman who is not immune |
✓ |
|
✓ |
|
21.9. Variations from the vaccine data sheet
See section 12.9 (external link) for variations from the MMR (Priorix) data sheet.
References
References
References
- Australian Technical Advisory Group on Immunisation. 2018. Australian Immunisation Handbook (ed.), Canberra: Australian Government Department of Health. URL: https://immunisationhandbook.health.gov.au/ (external link) (accessed October 2019)
- American Academy of Pediatrics. 2018. Rubella. in Red Book: 2018 Report of the Committee on Infectious Diseases, Kimberlin D, Brady M, Jackson M, et al. (eds). Elk Grove Village, IL. URL: https://redbook.solutions.aap.org/redbook.aspx (external link). (accessed 3 July 2020)
- Reef SE ,Plotkin S. 2018. Rubella Vaccines, in Plotkin's Vaccines (7th edition), Plotkin S, Orenstein W, Offit P, et al. (eds). Elsevier: Philadelphia, US.
- World Health Organization. Measles and Rubella Surveillance Data.: WHO; [updated 13 March 2020]; URL: https://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/active/measles_monthlydata/en/ (external link) (accessed 25 April 2020)
- Grant GB, Desai S, Dumolard L, et al. Progress toward rubella and congenital rubella syndrome control and elimination – worldwide, 2000–2018. MMWR: Morbidity and Mortality Weekly Report, 2019. 68(39): p. 855-859.
- Davidkin I, Jokinen S, Broman M, et al. Persistence of measles, mumps, and rubella antibodies in an MMR-vaccinated cohort: a 20-year follow-up. Journal of Infectious Diseases, 2008. 197(7): p. 950-6.
- American Academy of Pediatrics. 2018. Measles. in Red Book: 2018 Report of the Committee on Infectious Diseases, Kimberlin D, Brady M, Jackson M, et al. (eds). Elk Grove Village, IL. p. 537-550. URL: https://redbook.solutions.aap.org/redbook.aspx (external link). (accessed 3 July 2020)
- Strebel P, Papania M, Gastañaduy P, et al. 2018. Measles Vaccine, in Plotkin's Vaccines (7th edition), Plotkin S, Orenstein W, Offit P, et al. (eds). Elsevier: Philadelphia, US.
- Public Health England. 2016 Rubella susceptibility screening in pregnancy to end in England (Press release). Public Health England. 27 January 2016 URL: https://www.gov.uk/government/news/rubella-susceptibility-screening-in-pregnancy-to-end-in-england (external link). (accessed 10 May 2022)
- Peltola H ,Heinonen OP. Frequency of true adverse reactions to measles-mumps-rubella vaccine. A double-blind placebo-controlled trial in twins. Lancet, 1986. 1(8487): p. 939-42.
- Institute of Medicine: Committee to Review Adverse Effects of Vaccines. 2012. Adverse effects of vaccines: Evidence and causality (ed.), Washington, DC: The National Academies Press. URL: https://www.nap.edu/catalog/13164/adverse-effects-of-vaccines-evidence-and-causality (external link) (accessed January 2020)
- McLean HQ, Fiebelkorn AP, Temte JL, et al. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR: Recommendations and Reports, 2013. 62(RR-04): p. 1-34.