On this page
Key information
|
Mode of transmission |
Faecal–oral route through close personal contact and fomites. |
|---|---|
|
Incubation period |
1–3 days. |
|
Period of communicability |
During symptoms and until approximately 8 days after onset of symptoms. Up to 30 days after onset of symptoms in immunocompromised patients. |
|
Burden of disease |
All children during infancy or early childhood. Severe disease occurs most often in children aged 3 months to 2 years. |
|
Funded vaccine |
Oral live attenuated monovalent rotavirus: RV1 (Rotarix). |
|
Dose, presentation, route |
1.5 mL per dose. Oral suspension in an oral applicator, Administered orally. |
|
Funded vaccine indications and schedule |
2 doses for infants, at ages 6 weeks and 3 months. For catch-up schedules, the first dose should be given before age 15 weeks (latest is 14 weeks and 6 days), and the 2nd dose should be given before age 25 weeks (latest is 24 weeks and 6 days). |
|
Vaccine effectiveness |
Highly effective against severe rotavirus diarrhoea; some evidence for efficacy against all‑cause diarrhoea and herd protection. |
|
Contraindications |
Previous intussusception and with conditions that predispose the infant to intussusception. Severe combined immune deficiency. |
|
Precautions and special considerations |
Infants living in households with immunocompromised persons or pregnant women should still be vaccinated. Hand washing and the careful disposal of soiled nappies are likely to minimise any risk of vaccine transmission to household members. Immunosuppressed infants including those on immunosuppressive therapy (other than SCID, where it is contraindicated). Infants born to mothers on immunosuppressive biologic agents (see section 20.6.2). |
|
Potential responses to vaccine |
A possible, very small risk for intussusception; the benefits of immunisation considerably outweigh this potential risk. |
20.1. Virology
The rotaviruses are segmented, double-stranded RNA viruses of the family Reoviridae.[1] They are classified according to two surface proteins on the outer capsid: VP4 protease cleaved ‘P’ protein and VP7, the ‘G’ glycoprotein, which allows a binary classification system. The G and P proteins are immunological targets for neutralising antibodies protecting against disease and re-infection.[2] While more than 60 G and P combinations have been found in humans, there are only five strains (P[8]G1, P[4]G2, P[8]G3, P[8]G4, and P[8]G9) that are associated with 80–90 percent of the global burden of disease in children.[3] For simplicity, these strains are commonly referred to by their G serotype as G1, G2, G3, G4 and G9.
20.2. Clinical features
Rotavirus infects almost all children during infancy or early childhood. Transmission occurs through the faecal–oral route through close personal contact and through fomites. Aerosol transmission has been hypothesised but remains unproven.[1]
The incubation period is one to three days, after which illness can begin abruptly, with fever and vomiting often preceding the onset of diarrhoea.[1, 4] Up to one-third of children will develop a fever of greater than 39°C.[5, 6] The illness lasts from three to eight days.
Children with rotavirus are infectious while they have symptoms and until approximately eight days after the onset of symptoms. Immunocompromised patients may be infectious for up to 30 days after the onset of symptoms.[7] Large quantities of rotavirus are shed in the stool, and only a few virions are required to cause infection in a susceptible host.[8]
Rotavirus infection in the first three months of life is frequently mild or asymptomatic. This is possibly due to passive protection from maternally acquired antibodies, being breastfed and the intestinal cell structure of newborn infants.[1, 9]
The burden of severe dehydrating gastroenteritis caused by rotavirus occurs predominantly in infants and children aged 3 months to 2 years.[3]
The clinical spectrum ranges from asymptomatic infection to an acute severe illness with frequent and large-volume diarrhoea and vomiting, leading to dehydration, electrolyte disturbance and their sequelae. The illness spectrum from rotavirus is more severe than from other common causes of diarrhoea in children.[1]
20.3. Epidemiology
20.3.1. Global burden of disease
20.3.1. Global burden of disease
Rotavirus gastroenteritis is a significant cause of infant diarrhoea worldwide, both in high- and low-income countries. Virtually all children are infected by age 5 years.[1] Each year rotavirus causes the death of approximately 200,000 to 450,000 children aged under 5 years worldwide[10, 11] and results in 2.4 million paediatric hospital admissions.[12] Virtually all of the deaths occur in low-income countries. Prior to the introduction of licensed rotavirus vaccines in high-income countries, more than 220,000 children were hospitalised with rotavirus gastroenteritis every year.[13, 14]
Rates of rotavirus illness in children before the introduction of vaccine were similar in high- and low-income countries, indicating that good hygiene and clean water supplies are unlikely to have a significant impact on disease prevention. As a result, immunisation is the primary public health measure for the reduction of rotavirus disease burden.[1]
In countries with a temperate climate, rotavirus epidemics occur every winter and spring. Factors associated with an increased risk of severe rotavirus gastroenteritis include age under 2 years, low birthweight, premature gestation, lack of breastfeeding, socioeconomic disadvantage, malnutrition and impaired immunity.[1, 15, 16, 17, 18] Rotavirus gastroenteritis is not, however, more severe in HIV-infected children, although viral shedding may be longer.[3]
Rotavirus is an important cause of hospital-acquired infection[19] and can also cause disease in adults, especially those caring for children[20] and those living in aged-care facilities. During outbreaks in early childhood settings, rotavirus has been isolated from telephone receivers, drinking fountains, water-play tables and toilet handles.[21] Outbreaks in elderly populations may be linked to waning immunity, institutional crowding or both.
Children and adults can be infected with rotavirus several times in their lives. After a single natural infection during infancy, approximately one‑third are protected against subsequent rotavirus infection, more than three-quarters are protected against subsequent rotavirus gastroenteritis and 85–90 percent are protected against severe rotavirus gastroenteritis.[22] The proportion with protection against both infection and symptomatic rotavirus gastroenteritis increases with successive episodes.[22]
These observations serve as the biological basis for rotavirus vaccines, whereby live attenuated strains can induce cumulative protective immunity similar to that following natural infection by wild-type rotaviruses. Although the immune mechanism and correlates of protection against rotavirus infection are incompletely understood, it is likely that both mucosal and serum antibodies are associated with protection against rotavirus infection and disease.[23]
Since the introduction of the vaccine in other high-income countries, there have been reductions in all-cause and rotavirus gastroenteritis in age groups not eligible for the vaccine, suggesting herd immunity effects as a result of rotavirus vaccines[3, 24, 25 (external link)] (see ‘Herd immunity in the post-licensure period (external link)’ in section 20.4.2).
20.3.2. New Zealand epidemiology
20.3.2. New Zealand epidemiology
Rotavirus vaccine was introduced in July 2014, as a three-dose schedule to infants at ages 6 weeks, 3 months and 5 months, using the RV5 vaccine (RotaTeq) (see Appendix 1).
At present rotavirus is not a notifiable disease, so there is no national surveillance data available. National hospital discharge rates, community and hospital laboratory data plus a sentinel hospital-based surveillance system have been used to monitor rotavirus disease since vaccine introduction. The sentinel hospital-based rotavirus surveillance was introduced in December 2014 at Kidz First Children’s Hospital in Counties Manukau district and extended to Wellington, Hutt and Christchurch Hospitals in April 2016.
For detailed information about rotavirus surveillance and rotavirus infections in New Zealand, see the PHF Science (formerly ESR) website (external link).
Pre-vaccine epidemiology
Prior to the introduction of vaccine, by the age of 5 years, it is estimated 1 in 5 children had sought medical advice for rotavirus gastroenteritis and 1 in 43 children had been hospitalised.[13]
From 2010 to 2014, the average annual national hospitalisation rate for rotavirus in children aged under 5 years was 215.4 per 100,000.[26] The highest hospitalisation rates for children aged under 5 years were in those from the Middle Eastern/Latin American/African ethnic group, followed by Pacific and Māori ethnic groups. Hospitalisation rates in children aged under 5 years who reside in the most deprived NZDep2013 quintiles (quintiles 4 and 5) were significantly higher than those who reside in the least deprived quintile. There is a seasonal peak for rotavirus hospitalisations, usually occurring around September each year.
Post-vaccine epidemiology
The introduction of rotavirus vaccination in Australia resulted in a 70 percent decrease in rotavirus hospitalisations in the two and a half years post-vaccine introduction.[27 (external link)]
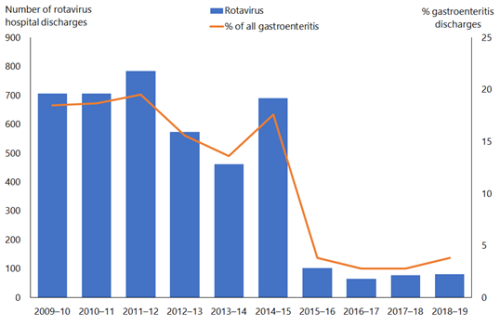
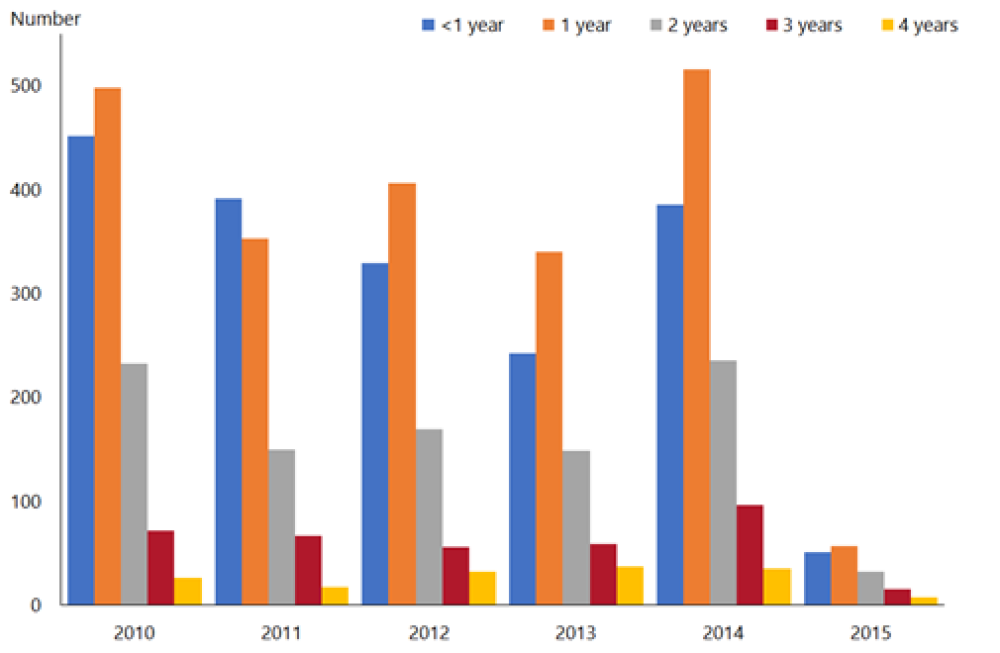
A similar decline has been noted in New Zealand in the first year post-vaccine introduction, where rotavirus hospitalisation rates for children aged under 5 years declined by 85 percent in 2015 compared with the previous five-year average (2010–2014)[26] (Figure 20.1). The vaccine has been effective in decreasing the most severe rotavirus disease. Hospitalisation rates decreased for all ethnic groups and levels of socioeconomic deprivation. Community laboratory data also supports the large decrease in rotavirus infections in the community.
Although only children aged under 1 year were eligible for rotavirus vaccination, hospital discharge rates decreased in all children aged under 5 years in 2015[26] (Figure 20.2). Older children are more likely to have been exposed to rotavirus already, and are less likely to benefit from vaccination.
Hospital discharges for rotavirus ranged from 510 to 822 cases per year in the four years prior to vaccine introduction (2010–2013).[26] There were 99 hospital discharges for rotavirus in children aged under 5 years in New Zealand in 2015, compared with 770 in 2014. This reduction in rotavirus hospitalisations of children aged under 5 years was maintained to 2019, with 80 cases hospitalised in the year to June 2019.
There was a 93.6 percent decrease in rotavirus outbreaks (three outbreaks reported in 2015 compared with 47 in 2014) after the introduction of vaccine in New Zealand.[26] This demonstrates that universal rotavirus vaccination is an effective public health intervention.
Figure 20.1: Rotavirus hospital discharges and as a percentage of all gastroenteritis discharges for children aged under 5 years, all New Zealand, June 2009–June 2019
|
Note: Rotavirus vaccine introduced in July 2014. Source: Ministry of Health. |
Figure 20.2: Rotavirus hospital discharge rates for children aged under 5 years by age and year, all New Zealand, 2010–2015
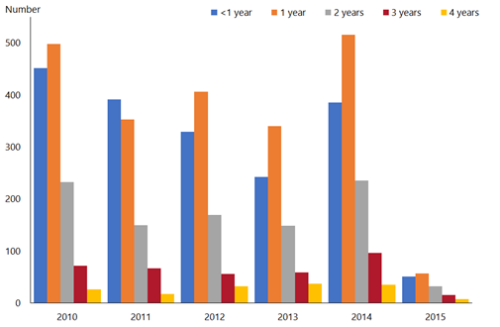
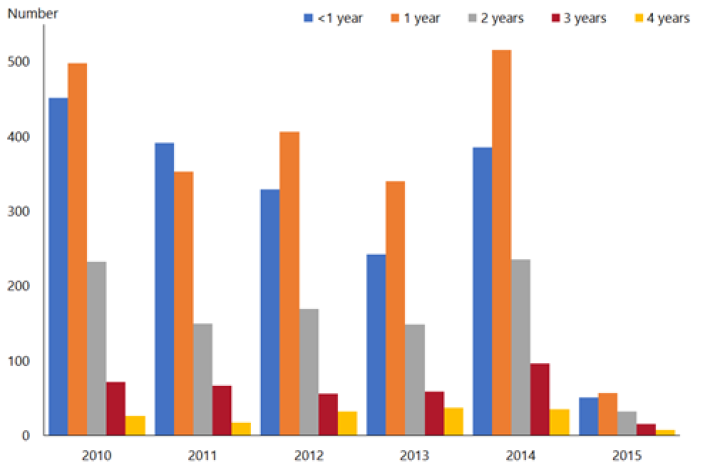
| Source: ESR |
20.4. Vaccines
20.4.1. Available vaccines
20.4.1. Available vaccines
The types of virus assessed for use as rotavirus vaccines have included live attenuated virus, both human and animal strains of the virus, and human–animal reassortant viruses. Two rotavirus vaccines have been used in New Zealand. Both are orally administered live attenuated vaccines and have been extensively evaluated.[28, 29] The live attenuated vaccine viruses replicate in the intestinal mucosa and are shed in the stools of vaccine recipients.[28, 30, 31 (external link)]
Funded vaccine
RV1 (Rotarix, GSK) is a live attenuated monovalent human G1P1A[8] strain rotavirus vaccine. It protects against non-G1 serotypes (these include G2P[4], G3P[8], G8P[4], G9P[8] and G12P[6]) on the basis of other shared epitopes. Each 1.5 mL dose contains:
- at least 106 CCID50 (cell culture infective dose 50 percent) of the RIX 4414 strain of human rotavirus
- other components and residuals, including sucrose, disodium adipate and culture medium.
20.4.2. Efficacy and effectiveness
20.4.2. Efficacy and effectiveness
Prevention of disease
A 2012 Cochrane review[32] of the efficacy of rotavirus vaccines for the prevention of rotavirus diarrhoea assessed 41 trials which met the inclusion criteria, involving 186,263 enrolled participants. Of these, 29 trials assessed the monovalent vaccine (RV1; Rotarix) and 12 trials assessed the pentavalent vaccine (RV5; RotaTeq).
For the first two years of life in countries with low mortality rates, both vaccines prevented over 80 percent of cases of severe rotavirus diarrhoea (Table 20.1). Both vaccines impact severe all-cause diarrhoea (moderate to low quality of evidence). See also Figure 20.1 and Figure 20.2 above, which show a reduction in rotavirus hospitalisations in New Zealand children aged under 5 years after rotavirus vaccine was introduced in 2014.
Table 20.1: Cochrane review: percentage of severe rotavirus and all-cause diarrhoea cases prevented in children by RV1 and RV5, compared to placebo (low mortality rate countries)
|
Vaccine |
Percentage of cases prevented |
Risk ratio |
Number of participants (number of trials) |
Quality of evidence |
|---|---|---|---|---|
|
Severe rotavirus diarrhoea: infants aged under 1 year |
||||
|
RV1 |
86 |
0.14 |
40,631 |
High |
|
RV5 |
87 |
0.13 |
2,344 |
Moderate |
|
Severe rotavirus diarrhoea: children aged under 2 years |
||||
|
RV1 |
85 |
0.15 |
32,854 |
High |
|
RV5 |
82 |
0.18 |
3,190 |
Moderate |
|
Severe all-cause diarrhoea: infants aged under 1 year |
||||
|
RV1 |
40 |
0.60 |
17,867 |
Moderate |
|
RV5 |
72 |
0.28 |
1,029 |
Low |
|
Severe all-cause diarrhoea: children aged under 2 years |
||||
|
RV1 |
37 |
0.63 |
39,091 |
Moderate |
|
RV5 |
96 |
0.04 |
5,916 |
Low |
| Adapted from: Soares-Weiser K, MacLehose H, Bergman H, et al. 2012. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database of Systematic Reviews Issue 11, Art. No. CD008521. DOI: 10.1002/14651858.CD008521.pub3 (accessed 24 June 2020). | ||||
Effectiveness
In pre-marketing clinical trials, rotavirus vaccination prevented 42–58 percent of all-cause hospital admissions for acute gastroenteritis, suggesting it is responsible for more gastroenteritis than is detected by routine testing.[28, 33, 34]
Post-licensure surveillance studies have demonstrated large reductions in rotavirus-positive stool isolates from children with gastroenteritis (US)[35] and in diarrhoea-related deaths (Mexico).[36, 37] Summarised, post-licensure vaccine effectiveness studies in high-income countries have shown an 89–100 percent reduction in emergency department visits or hospitalisation; a 74–90 percent decline in hospitalisations for rotavirus gastroenteritis in children aged under 2 years; and a 29–50 percent decline in ‘all‑cause’ acute gastroenteritis hospitalisations for children aged under 5 years.[38]
A protective association between rotavirus vaccine and childhood seizures has been reported in the US[39] and Australia.[40] In US children, a full course of rotavirus vaccination was associated with an 18–21 percent reduction in the risk of seizure requiring hospitalisation or emergency department care in the year following vaccination, compared with unvaccinated children.[39] In the Australian state of Queensland, rotavirus vaccine was 35.8 percent effective at preventing emergency department presentation for febrile seizures and 38.0 percent effective at preventing subsequent hospitalisation in children up to two years following vaccination.[40]
Herd immunity in the post-licensure period
Since the beginning of the post-licensure period, over 80 countries have introduced the rotavirus vaccine into their national immunisation programmes.[41] There has been substantial though somewhat variable efficacy data to show a decline in rotavirus infections in the different country environments. In the US, there was a 73 percent reduction in rotavirus infections among infants from 2003 to 2014.[42] The effectiveness tends to wane with age, and rotavirus ‘seasons’ appear to be longer in the post-licensure period.[42]
While a decline has occurred in rotavirus infection alone, there has also been a reduction in all-cause diarrhoeal illnesses.[41] Furthermore, the protective effect of the vaccine has surpassed the expected level of vaccine efficacy and coverage, resulting in a herd protection. Therefore, the immunised proportion of the population is causing a reduction of infection in the unimmunised portion of the community.[43]
Duration of protection
Prior to the introduction of rotavirus vaccines in Europe, extension studies of the pivotal phase III RV5 trial showed protection lasting up to three years from the last vaccine dose.[44] The duration of protection provided by rotavirus vaccines is difficult to measure because of the herd immunity effect that occurs after the vaccine is implemented. Some studies indicate waning immunity after the first year of life, particularly in low-income countries.[45, 46] In a large multicentre study in the US, both RV1 and RV5 vaccines were found to provide lasting and broadly heterologous protection against infection. Vaccine effectiveness persisted to the seventh year of life for RV5 and through the third year of life for RV1.[47] Note that the differences in duration are because RV1 was licensed in the US approximately two years later than RV5, affecting vaccination coverage and corresponding study power for older age groups for RV1 analyses.[47]
Partial vaccination
Studies in partially vaccinated infants (ie, those who had not completed the three-dose course of RV5 or the two-dose course of RV1) found that protection against rotavirus gastroenteritis ranged from 51–55 percent in low- and middle-income countries, and from 69–93 percent in high-income countries.[48]
Cross-protection
Rotavirus vaccine strains vary considerably, and multiple wild-type strains can occur at the same time. In high-income countries, both vaccines appear to provide some cross-protection against non-vaccine serotypes.[49, 50] Vaccine protection against newly emerging genotypes is not well known, and national surveillance of circulating rotavirus types post-vaccination is necessary.[51]
20.4.3. Transport, storage and handling
20.4.3. Transport, storage and handling
Transport according to the National Standards for Vaccine Storage and Transportation for Immunisation Providers 2017 (2nd edition) (external link).
Store in the dark at +2°C to +8°C. Do not freeze.
20.4.4. Dosage and administration
20.4.4. Dosage and administration
The dose of RV1 (Rotarix) is 1.5 mL, administered orally (for administration instructions see section A7.2.4 (external link) of this Handbook or the vaccine data sheet, available on the Medsafe website (external link)). Do not inject RV1.
Two doses are given, at ages 6 weeks and 3 months. See section 20.5 (external link) below for more information.
Co-administration with other vaccines
Rotavirus vaccines can be administered at the same time as other scheduled vaccines. Note that no time interval is required between administration of rotavirus and BCG vaccines; the two live vaccines likely to be administered to infants aged under 6 months.
If the dose is regurgitated or vomited
If the dose of rotavirus vaccine is regurgitated or vomited during or after administration, a repeat dose should not be given.[52] The second dose should be administered as per the schedule. Receptor binding of vaccine is instantaneous, making repeat dosing unnecessary.
If the first dose is immediately spat out then a single repeat dose could be given.
20.5. Recommended immunisation schedule
RV1 is recommended and funded for all infants. See section 20.5.2 (external link) for RV1 age limit information.
Immunisation is especially encouraged for those who will be attending early childhood education services or where there is an immunocompromised individual living in the household.
Infants who have already had rotavirus gastroenteritis should still receive the full course of immunisation. Initial rotavirus infection only provides partial protection against subsequent infection.[1, 22]
20.5.1. Routine schedule
20.5.1. Routine schedule
Two RV1 doses are given orally, at ages 6 weeks and 3 months.
Table 20.2: The infant RV1 (Rotarix) schedule
|
Dose |
Usual scheduled age |
Recommended age limits for dosing |
|---|---|---|
|
Dose 1 |
6 weeks |
6–14 weeksa |
|
Dose 2b |
3 months |
10–24 weeksc |
|
a. The upper age limit for receipt of the first dose of RV1 is immediately prior to turning 15 weeks old (14 weeks and 6 days). b. The minimum interval between doses 1 and 2 is 4 weeks. c. The upper age limit for receipt of the second dose of RV1 is immediately prior to turning 25 weeks old (24 weeks and 6 days). |
||
20.5.2. Catch-up schedules
20.5.2. Catch-up schedules
The first dose of RV1 should be given before age 15 weeks (ie, 14 weeks and 6 days), and the second dose administered at least four weeks later (see Table 20.2 (external link)). An infant who has not had the first dose before age 15 weeks will not be able to commence the rotavirus course. Where the first dose is inadvertently given at age 15 weeks or older, the second dose should be given, but both doses should be given before age 25 weeks (ie, the latest is 24 weeks and 6 days).[1] Rotavirus vaccine is not intended for use in older children, adolescents or adults.
The age limits for initiating and completing the vaccine series are recommended because there is insufficient safety data on the use of these vaccines outside this age range. If a partially vaccinated infant reaches age 25 weeks before the second dose is given, the first dose already given will offer them partial protection against disease.
The severity of rotavirus infection decreases with age, so a cost–benefit analysis for vaccinating older children is a low priority and has not been done.
20.5.3. Preterm infants
20.5.3. Preterm infants
Vaccination as per the Schedule (ie, at the usual chronological age, with the usual vaccine dosage and interval) is recommended for preterm infants and infants with low birthweight, including those still in hospital (see below). Rotavirus vaccine can be given to preterm infants born who are receiving corticosteroids. (See also 4.2.1 (external link) for more immunisation recommendations for preterm infants.)
20.5.4. Hospitalised infants
20.5.4. Hospitalised infants
Rotavirus vaccine should be given on time to any infant admitted to a general hospital ward (where other patients are not high risk). If standard infection control precautions are maintained, there is no risk of transmission of vaccine strain rotavirus when rotavirus vaccine is administered to hospitalised infants, including hospitalised preterm infants and those in neonatal units.[53, 54] (See also section 4.2 for more information about infants with special immunisation recommendations.)
20.5.5. Pregnancy and breastfeeding
20.5.5. Pregnancy and breastfeeding
There is no concern caused by vaccine exposure during pregnancy. There is no restriction for breastfeeding before or after vaccination of the infant (see ‘Shedding (external link)’ in section 20.6.2).
20.6. Contraindications and precautions
See section 2.1.3 for pre-vaccination screening guidelines and section 2.1.4 for general contraindications for all vaccines.
20.6.1. Contraindications
20.6.1. Contraindications
Rotavirus vaccine should not be given to infants with:
- a history of a severe (anaphylactic) allergic reaction after a previous dose or to a vaccine component
- a history of intussusception or an uncorrected congenital malformation of the gastrointestinal tract that would predispose the infant to intussusceptions (see section 20.7.1).
- severe combined immunodeficiency (SCID).[55]
20.6.2. Precautions
20.6.2. Precautions
Rotavirus vaccine can be administered to infants with a mild illness, including gastroenteritis and upper respiratory tract infections. Infants with moderate to severe gastroenteritis should not be vaccinated until symptoms resolve.
There is very little safety data on infants with predisposing conditions such as metabolic disorders and chronic gastrointestinal diseases (Hirschsprung’s, malabsorption syndromes or short gut syndromes). Since there is a greater risk of serious wild-type rotavirus disease, the benefits outweigh the risk, and vaccination is encouraged.[52]
Infants who have received antibody-containing blood products and are the appropriate age should be vaccinated. Rotavirus vaccine and antibody-containing blood products can be administered simultaneously.[52] There is a theoretical risk of interference in the immune response to the vaccine; therefore the interval between vaccination and receipt of blood products should ideally be as long as possible within the age limits of the vaccine schedule.
Administration of RV1 in immunosuppressed infants, including infants on immunosuppressive therapy, should be based on careful consideration of potential benefits and risks.
Infants born to mothers on immunosuppressive therapies
There is limited data on rotavirus vaccination safety when given to infants born to mothers receiving immunosuppressive therapy during pregnancy.[19, 36, 56 (external link)] Although in most cases it is likely to be safe, caution is required. The level of circulating wild-type rotavirus is currently very low in New Zealand; therefore, the risk of gastroenteritis following rotavirus vaccination in this cohort of infants may be greater than the risk of acquiring the disease. The decision to administer rotavirus vaccine to infants born to mothers who received immunosuppressive agents (biologic agents) during pregnancy should be determined case by case.
If an infant turns 15 weeks of age before the first rotavirus vaccine dose can be administered, they will not be able to receive any rotavirus vaccine doses.
See section 4.2.5 'Infants of mothers who received immunomodulatory biologic agents during pregnancy' and Table 4.2 (external link) for a list of the highly immunomodulatory biologic agents with long half-lives that require a prolonged delay before vaccination (for up to one year in those being treated). These include monoclonal antibody (mab) agents that readily cross the placenta.
Each case should be assessed on a risk–benefit basis and with specialist advice.
Shedding
Since rotavirus vaccine virus replicates in the gastrointestinal tract, it can be shed in stools – especially after the first dose.[57] Shedding is also more likely in immunocompromised patients (eg, children with HIV). The vaccine virus could then be transmitted to unvaccinated populations; a feature that is generally beneficial as it promotes herd immunity.
Infants living in households with immunocompromised individuals should be vaccinated. So far there are no safety concerns, but there is also no data to confirm the safety of these vaccines for immunocompromised patients. Infants living in households with pregnant women should also be vaccinated. Hand washing and the careful disposal of soiled nappies are likely to minimise any risk of vaccine transmission to household members.[52, 53]
20.7. Potential responses and AEFIs
The 2012 Cochrane review[32 (external link)] described in section 20.4.2 (external link) also reviewed the safety of RV1 and RV5 vaccines. No significant difference was found between children receiving RV1 or RV5 and placebo in the number of serious adverse events, particularly intussusception (see below). No statistical differences were observed for fever, diarrhoea and vomiting between cases and placebo groups. There was no significant difference between cases and placebos in the number of adverse events leading to discontinuation of the schedule.
In 2010 porcine circovirus or porcine circovirus DNA was detected in both rotavirus vaccines. However, there is no evidence that this virus is a safety risk or causes illness in humans.[52 (external link)]
20.7.1. Intussusception
20.7.1. Intussusception
Intussusception is a cause of an acute abdomen when one part of the intestine telescopes into another part of the intestine; the mechanism by which these events occur remains uncertain. In 1999 an oral human–rhesus rotavirus quadrivalent vaccine (RotaShield) was licensed in the US and on the infant schedule but was withdrawn later that year after reports of an association with intussusception (a risk of approximately one case in 5,000–10,000 vaccine recipients).
No increased risk of intussusception was detected in the large phase III pre-licensure clinical trials of RV1 (Rotarix) and RV5 (RotaTeq), despite this being a specifically monitored adverse event. However, post-marketing surveillance of both rotavirus vaccines indicates the possibility of an increased risk of intussusception shortly after the first dose of rotavirus vaccination. Evidence from Australia[58] indicates that after the first dose, RV1 had a relative incidence (relative risk) of 6.8 (95% CI: 2.4–19.0, p<0.001) and 3.5 (95% CI: 1.3–8.9, p=0.01) for the periods of 1–7 days and 8–21 days after vaccination, respectively. For RV5, the relative incidence was 9.9 (95% CI: 3.7–26.4, p<0.001) and 6.3 (95% CI: 2.8–14.4, p<0.001) for the same time periods.
There was also some elevated risk of intussusception 1 to 7 days after the second dose of both vaccines. The relative incidence for RV1 was 2.8 (95% CI: 1.1–7.3, p=0.03) and for RV5 was 2.8 (95% CI: 1.2–6.8, p=0.02). There was no evidence of increased risk of intussusception following a third dose of RV5.[58] The increased risk of intussusception following rotavirus vaccination is estimated at approximately 6 additional cases of intussusception among every 100,000 infants vaccinated (approximately 1 in 15,500 vaccine recipientss), or 14 additional cases per year in Australia.[58]
Studies in the post-licensure period continue to show small increases in risk for both RV1 and RV5 and primarily within seven days of the first dose of vaccine.[59, 60] Recent safety data has continued to emphasise the clear and dramatic benefit of vaccination over the very low risk of vaccine-associated intussusception.[41] For example, a self-controlled case-series study estimated that the RV1 programme in England caused 21 intussusception admissions annually and prevented 25,000 gastrointestinal infection admissions with a clear risk–benefit ratio.[61]
While there appears to be an increased relative risk of intussusception, the condition remains rare, and this risk is outweighed by the benefits of rotavirus vaccination in preventing rotavirus infections; there was an estimated 70 percent reduction in hospitalisations in young children after the vaccine’s introduction to the Australian schedule.[62] It is uncertain whether rotavirus vaccine administration affects the overall incidence of intussusception: US data suggests no increased overall rate in infants despite a small cluster effect.[63] Both the WHO[64] and the Australian Technical Advisory Group on Immunisation[62] continue to recommend the use of rotavirus vaccine for infants.
Although the risk of intussusception after rotavirus immunisation is very small, it is recommended that parents seek medical advice and health care professionals are attentive if the baby develops intermittent crying or screaming episodes, pulling their knees towards their chest and vomiting, or pink- or red-coloured jelly-like stools.
A recent study has described the epidemiology of intussusception in New Zealand children aged 0–36 months (794 cases) for a 16-year period before the introduction of routine rotavirus vaccination.[65 (external link)] This study will provide a valuable baseline to determine if the introduction of the vaccine has significant effects on intussusception rates in the New Zealand population.
20.8. Public health measures
Prevention of spread is by contact precautions, including careful handwashing. In an early childhood service setting where there has been a child known to have had a rotavirus infection, the surfaces should be washed with sodium hypochlorite (bleach) and water. Disinfectants inactivate rotavirus and may help to prevent disease transmission resulting from contact with environmental surfaces.[52]
For more details on control measures, refer to the ‘Acute gastroenteritis’ chapter of the Communicable Disease Control Manual.
20.9. Variations from the vaccine data sheet
The RV1 data sheet recommends postponing the administration of the vaccine in infants suffering from diarrhoea or vomiting. Health NZ recommends vaccinating infants with mild gastroenteritis, and to wait until symptoms have resolved for infants with moderate to severe gastroenteritis (see section 20.6.2 (external link)).
The RV1 data sheet states that the vaccine should not be administered to subjects with any chronic gastrointestinal disease. Health NZ recommends instead that pre-existing chronic gastrointestinal disease is not a contraindication to rotavirus vaccination, with the exception of those conditions that may predispose the infant to intussusceptions (see sections 20.6.1 (external link) and 20.7.1).[53]
References
References
References
- Cortese MM, Parashar UD, Centers for Disease C, et al. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR: Recommendations and Reports, 2009. 58(RR-2): p. 1-25.
- Cunliffe NA ,Nakagomi O. A critical time for rotavirus vaccines: a review. Expert Rev Vaccines, 2005. 4(4): p. 521-32.
- Parashar U, Cortese MM ,Offit P. 2018. Rotavirus vaccines, in Plotkin's Vaccines, 7th edition, Plotkin S, Orenstein W, Offit P, et al. (eds). Elsevier: Philadelphia, US.
- Parashar UD, Nelson EA ,Kang G. Diagnosis, management, and prevention of rotavirus gastroenteritis in children. BMJ, 2013. 347(30 December): p. f7204.
- Rodriguez WJ, Kim HW, Arrobio JO, et al. Clinical features of acute gastroenteritis associated with human reovirus-like agent in infants and young children. Journal of Pediatrics, 1977. 91(2): p. 188-93.
- Ruuska T ,Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scandinavian Journal of Infectious Diseases, 1990. 22(3): p. 259-67.
- Ministry of Health. 2012. Communicable Disease Control Manual (ed.), Wellington: Ministry of Health. URL: https://www.health.govt.nz/publication/communicable-disease-control-manual (external link) (accessed 10 May 2022)
- Bishop RF. Natural history of human rotavirus infection. Archives of Virology. Supplementum, 1996. 12: p. 119-28.
- Bishop RF, Barnes GL, Cipriani E, et al. Clinical immunity after neonatal rotavirus infection. A prospective longitudinal study in young children. New England Journal of Medicine, 1983. 309(2): p. 72-6.
- Tate JE, Burton AH, Boschi-Pinto C, et al. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infectious Diseases, 2012. 12(2): p. 136-41.
- Fischer Walker CL, Rudan I, Liu L, et al. Global burden of childhood pneumonia and diarrhoea. The Lancet, 2013. 381(9875): p. 1405-1416.
- Grimwood K ,Lambert SB. Rotavirus vaccines: opportunities and challenges. Hum Vaccin, 2009. 5(2): p. 57-69.
- Milne RJ ,Grimwood K. Budget impact and cost-effectiveness of including a pentavalent rotavirus vaccine in the New Zealand childhood immunization schedule. Value in Health, 2009. 12(6): p. 888-98.
- Parashar UD, Hummelman EG, Bresee JS, et al. Global illness and deaths caused by rotavirus disease in children. Emerging Infectious Diseases, 2003. 9(5): p. 565-72.
- Dennehy PH, Cortese MM, Begue RE, et al. A case-control study to determine risk factors for hospitalization for rotavirus gastroenteritis in U.S. children. Pediatric Infectious Disease Journal, 2006. 25(12): p. 1123-31.
- Huppertz H-I, Salman N ,Giaquinto C. Risk factors for severe rotavirus gastroenteritis. Pediatric Infectious Disease Journal, 2008. 27(1): p. S11–19.
- Newman RD, Grupp-Phelan J, Shay DK, et al. Perinatal risk factors for infant hospitalization with viral gastroenteritis. Pediatrics, 1999. 103(1): p. E3.
- Sethi D, Cumberland P, Hudson MJ, et al. A study of infectious intestinal disease in England: risk factors associated with group A rotavirus in children. Epidemiology and Infection, 2001. 126(1): p. 63-70.
- Chandran A, Heinzen RR, Santosham M, et al. Nosocomial rotavirus infections: a systematic review. Journal of Pediatrics, 2006. 149(4): p. 441-7.
- Grimwood K, Abbott GD, Fergusson DM, et al. Spread of rotavirus within families: a community based study. British Medical Journal (Clinical Research Ed.), 1983. 287(6392): p. 575-7.
- Butz AM, Fosarelli P, Dick J, et al. Prevalence of rotavirus on high-risk fomites in day-care facilities. Pediatrics, 1993. 92(2): p. 202-5.
- Velázquez FR, Matson DO, Calva JJ, et al. Rotavirus infection in infants as protection against subsequent infections. New England Journal of Medicine, 1996. 335(14): p. 1022-8.
- Angel J, Franco MA ,Greenberg HB. Rotavirus immune responses and correlates of protection. Current Opinion in Virology, 2012. 2(4): p. 419-25.
- Buttery JP, Lambert SB, Grimwood K, et al. Reduction in rotavirus-associated acute gastroenteritis following introduction of rotavirus vaccine into Australia's National Childhood vaccine schedule. Pediatric Infectious Disease Journal, 2011. 30(1 Suppl): p. S25-9.
- Parashar UD, Johnson H, Steele AD, et al. Health impact of rotavirus vaccination in developing countries: progress and way forward. Clinical Infectious Diseases, 2016. 62 Suppl 2(Supplement 2): p. S91-5.
- Institute of Environmental Science and Research Ltd. 2016. Rotavirus in New Zealand, 2015 (ed.), Porirua: Institute of Environmental Science and Research Ltd. URL: https://surv.esr.cri.nz/PDF_surveillance/Rotavirus/2015Rotavirus.pdf (external link) (accessed 19 January 2017)
- Macartney KK, Porwal M, Dalton D, et al. Decline in rotavirus hospitalisations following introduction of Australia's national rotavirus immunisation programme. Journal of Paediatrics and Child Health, 2011. 47(5): p. 266-70.
- Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. New England Journal of Medicine, 2006. 354(1): p. 23-33.
- Vesikari T, Karvonen A, Prymula R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet, 2007. 370(9601): p. 1757-63.
- Phua KB, Quak SH, Lee BW, et al. Evaluation of RIX4414, a live, attenuated rotavirus vaccine, in a randomized, double-blind, placebo-controlled phase 2 trial involving 2464 Singaporean infants. Journal of Infectious Diseases, 2005. 192 Suppl 1(Suppl 1): p. S6-S16.
- Salinas B, Perez Schael I, Linhares AC, et al. Evaluation of safety, immunogenicity and efficacy of an attenuated rotavirus vaccine, RIX4414: A randomized, placebo-controlled trial in Latin American infants. Pediatric Infectious Disease Journal, 2005. 24(9): p. 807-16.
- Soares-Weiser K, Maclehose H, Bergman H, et al. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev, 2012. 11(Art. No. CD008521): p. CD008521.
- Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. New England Journal of Medicine, 2006. 354(1): p. 11-22.
- Vesikari T, Giaquinto C ,Huppertz HI. Clinical trials of rotavirus vaccines in Europe. Pediatric Infectious Disease Journal, 2006. 25(1 Suppl): p. S42-7.
- Clark HF, Offit PA, Parashar UD. 2013. Rotavirus vaccines, in Vaccines (Basel), Plotkin SA, Orenstein WA, Offit PA (eds) (eds). Elsevier Saunders: Philadelphia, PA.
- Richardson V, Hernandez-Pichardo J, Quintanar-Solares M, et al. Effect of rotavirus vaccination on death from childhood diarrhea in Mexico. New England Journal of Medicine, 2010. 362(4): p. 299-305.
- Gastañaduy PA, Sánchez-Uribe E, Esparza-Aguilar M, et al. Effect of rotavirus vaccine on diarrhea mortality in different socioeconomic regions of Mexico. Pediatrics, 2013. 131(4): p. e1115-20.
- Sheridan S, Lambert S ,Grimwood K. Impact of rotavirus vaccination on childhood gastroenteritis. Microbiology Australia, 2012. 33(May): p. 56–60.
- Payne DC, Baggs J, Zerr DM, et al. Protective association between rotavirus vaccination and childhood seizures in the year following vaccination in US children. Clinical Infectious Diseases, 2014. 58(2): p. 173-7.
- Sheridan SL, Ware RS, Grimwood K, et al. Febrile seizures in the era of rotavirus vaccine. J Pediatric Infect Dis Soc, 2016. 5(2): p. 206-9.
- Yen C, Healy K, Tate JE, et al. Rotavirus vaccination and intussusception - Science, surveillance, and safety: A review of evidence and recommendations for future research priorities in low and middle income countries. Human Vaccines & Immunotherapeutics, 2016. 12(10): p. 2580-2589.
- Kaufman HW ,Chen Z. Trends in Laboratory Rotavirus Detection: 2003 to 2014. Pediatrics, 2016. 138(4): p. 1–6.
- Pollard SL, Malpica-Llanos T, Friberg IK, et al. Estimating the herd immunity effect of rotavirus vaccine. Vaccine, 2015. 33(32): p. 3795-800.
- Vesikari T, Karvonen A, Ferrante SA, et al. Sustained efficacy of the pentavalent rotavirus vaccine, RV5, up to 3.1 years following the last dose of vaccine. Pediatric Infectious Disease Journal, 2010. 29(10): p. 957-63.
- Correia JB, Patel MM, Nakagomi O, et al. Effectiveness of monovalent rotavirus vaccine (Rotarix) against severe diarrhea caused by serotypically unrelated G2P[4] strains in Brazil. Journal of Infectious Diseases, 2010. 201(3): p. 363-9.
- Yen C, Figueroa JR, Uribe ES, et al. Monovalent rotavirus vaccine provides protection against an emerging fully heterotypic G9P[4] rotavirus strain in Mexico. Journal of Infectious Diseases, 2011. 204(5): p. 783-6.
- Payne DC, Selvarangan R, Azimi PH, et al. Long-term consistency in rotavirus vaccine protection: RV5 and RV1 vaccine effectiveness in US children, 2012–2013. Clinical Infectious Diseases, 2015. 61(12): p. 1792-9.
- Patel MM, Glass R, Desai R, et al. Fulfilling the promise of rotavirus vaccines: how far have we come since licensure? Lancet Infectious Diseases, 2012. 12(7): p. 561-70.
- Steele AD, Neuzil KM, Cunliffe NA, et al. Human rotavirus vaccine Rotarix provides protection against diverse circulating rotavirus strains in African infants: a randomized controlled trial. BMC Infectious Diseases, 2012. 12(213): p. 213.
- Armah GE, Sow SO, Breiman RF, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet, 2010. 376(9741): p. 606-14.
- Kirkwood CD. Genetic and antigenic diversity of human rotaviruses: potential impact on vaccination programs. Journal of Infectious Diseases, 2010. 202 Suppl(S1): p. S43-8.
- American Academy of Pediatrics. 2018. Rotavirus infections. in Red Book: 2018 Report of the Committee on Infectious Diseases, Kimberlin D, Brady M, Jackson M, et al. (eds). Elk Grove Village, IL. p. 700-705. URL: https://redbook.solutions.aap.org/redbook.aspx (external link). (accessed 3 July 2020)
- Australian Technical Advisory Group on Immunisation (ATAGI). 2018. Rotavirus. in Australian Immunisation Handbook. Canberra. URL: https://immunisationhandbook.health.gov.au/vaccine-preventable-diseases/rotavirus (external link). (accessed 25 April 2020)
- Sicard M, Bryant K, Muller ML, et al. Rotavirus vaccination in the neonatal intensive care units: where are we? A rapid review of recent evidence. Current Opinion in Pediatrics, 2020. 32(1): p. 167-191.
- Centers for Disease Control and Prevention. 2010. Addition of severe combined immunodeficiency as a contraindication for administration of rotavirus vaccine. Morbidity and Mortality Weekly Report. 59(22): p. 687–8. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5922a3.htm (external link) (accessed 30 May 2020)
- Østensen M. Safety issues of biologics in pregnant patients with rheumatic diseases. Annals of the New York Academy of Sciences, 2014. 1317(1): p. 32-8.
- Boom JA, Sahni LC, Payne DC, et al. Symptomatic infection and detection of vaccine and vaccine-reassortant rotavirus strains in 5 children: a case series. Journal of Infectious Diseases, 2012. 206(8): p. 1275-9.
- Carlin JB, Macartney KK, Lee KJ, et al. Intussusception risk and disease prevention associated with rotavirus vaccines in Australia's National Immunization Program. Clinical Infectious Diseases, 2013. 57(10): p. 1427-34.
- Tate JE, Yen C, Steiner CA, et al. Intussusception rates before and after the introduction of rotavirus vaccine. Pediatrics, 2016. 138(3): p. e20161082.
- Walter EB ,Staat MA. Rotavirus vaccine and intussusception hospitalizations. Pediatrics, 2016. 138(3): p. e20161952.
- Stowe J, Andrews N, Ladhani S, et al. The risk of intussusception following monovalent rotavirus vaccination in England: A self-controlled case-series evaluation. Vaccine, 2016. 34(32): p. 3684-9.
- Therapeutic Goods Administration. 2013 Rotavirus vaccination and the risk of intussusception. Australian Government, Department of Health; 2013 [updated 28 August 2013]; URL: https://www.tga.gov.au/safety/alerts-medicine-rotavirus-130828.htm (external link). (accessed 3 July 2020)
- Yen C, Tate JE, Steiner CA, et al. Trends in intussusception hospitalizations among US infants before and after implementation of the rotavirus vaccination program, 2000-2009. Journal of Infectious Diseases, 2012. 206(1): p. 41-8.
- World Health Organization. Position paper on rotavirus vaccines. Weekly Epidemiological Record, 2013. 88(5): p. 49–64.
- Rosie B, Dalziel S, Wilson E, et al. Epidemiology of intussusception in New Zealand pre-rotavirus vaccination. New Zealand Medical Journal, 2016. 129(1442): p. 36-45.