On this page
Key information
|
Mode of transmission |
Contact with respiratory droplets. |
|---|---|
|
Incubation period |
Asymptomatic nasopharyngeal carriage is common. The incubation period is variable and may be as short as 1–3 days. |
|
Incidence and burden of disease |
Highest at extremes of age (<2 years and >75 years), Māori and Pacific people, those with multiple comorbidities and with immunocompromise. |
|
Funded vaccines |
All children aged under 5 years: PCV13 (Prevenar 13). Children and adults with eligible conditions:
|
|
Dose, presentation and route |
All vaccines:
|
|
Funded vaccine indications and schedule |
PCV13 at ages 6 weeks, 5 months and 12 months, and age-appropriate catch-up for children <5 years; an additional dose is given at age 3 months for those with eligible conditions. PCV13 and 23PPV:
|
|
Vaccine efficacy |
For pneumococcal conjugate vaccines: reductions in pneumococcal disease and carriage of vaccine serotypes in vaccinated populations, plus herd immunity effects reducing pneumococcal disease in other age groups; some increases in disease caused by non-vaccine serotypes. |
|
Precautions and special considerations |
Concomitant PCV13 and influenza vaccine may increase risk of fever and febrile convulsions in children aged 6 months to <5 years. 23PPV should not be given to children aged under 2 years as polysaccharide vaccines are poorly immunogenic in this age group. |
|
Public health measures |
Notify the local medical officer of health immediately on suspicion of invasive pneumococcal disease (see section 17.8) |
|
Post-exposure prophylaxis |
Antimicrobial prophylaxis is not indicated. |
17.1. Bacteriology
Streptococcus pneumoniae is a gram-positive diplococcus. It is ubiquitous, and many individuals carry the organism asymptomatically in their upper respiratory tract.[1] There are over 90 identifiable serotypes of S. pneumoniae. Certain serotypes are more invasive or more associated with antibiotic resistance, and dominant serotypes vary by age and geographical distribution.
See section 17.4.1 and Table 17.1 for the serotypes contained in the pneumococcal conjugate vaccines (PCV) and pneumococcal polysaccharide vaccine (PPV).
17.2. Clinical features
Streptococcus pneumoniae causes a wide variety of clinical illnesses from common but troublesome otitis media to life-threatening invasive pneumococcal disease (IPD). IPD is defined by isolation of S. pneumoniae from a usually sterile site, such as blood, pleural fluid or cerebrospinal fluid, and represents the most severe end of the disease spectrum. The most common clinical syndromes in IPD are bacteraemic pneumonia, non-localised bacteraemia and meningitis. Older adults generally have bacteraemic pneumonia, while young children may have any of the three, with meningitis being the most severe.
Pneumonia without bacteraemia is up to five times more common than bacteraemic pneumonia, especially in older adults, where it also has high mortality. Non-invasive infections include acute otitis media (predominantly in children) and sinusitis (predominantly older children and adults).
17.3. Epidemiology
17.3.1. Global burden of disease
17.3.1. Global burden of disease
The human nasopharynx is the only natural reservoir of S. pneumoniae. Transmission is by contact with respiratory droplets. Carriage rates in young children range from 21 percent in high-income settings to more than 90 percent in resource-limited countries.[1] Although nasopharyngeal colonisation precedes disease, most who are colonised do not develop disease. The period between colonisation with S. pneumoniae and infection is variable but may be as short as one day. Transmission and invasive potential are increased by concomitant viral upper respiratory tract infection, especially influenza.
Pneumococcal disease is a common cause of morbidity and mortality worldwide. Rates of disease and death are highest in low-income countries with the majority of deaths occurring in sub-Saharan Africa and Asia.[2] Along with the very old and very young, patients with underlying cardiorespiratory disease and congenital or acquired immunosuppression have the highest rates of disease.
Risk of disease increases with multiple comorbidities and lifestyle factors (this is described as risk-stacking, see section 17.5.4).[3] The risk of IPD in children and adults with two or more comorbid conditions can be as high as in those with a recognised ‘high-risk’ condition.[4] Lifestyle factors, such as passive smoking, environmental and workplace pollutions, smoking and alcohol dependency, can increase the risk of severe pneumococcal disease, especially in those with chronic illnesses that predispose them to infection, such as asthma, diabetes, dementia and mental illness.[5, 6] Socioeconomic deprivation, homelessness and overcrowding have also been associated with increased risk of IPD.[7]
The WHO estimates that 300,000 (range 200–370,000) children aged under 5 years died from pneumococcal infections, representing around 5 percent of all-cause mortality in this age group, in 2015.[8] An additional 23,000 (15–40,000) deaths were estimated to occur in children co-infected with HIV. On average 75 percent of IPD and 83 percent of pneumococcal meningitis cases are aged under 2 years but the incidence and age distribution vary by country and socioeconomic status.[8] Importantly, at least one quarter of survivors of pneumococcal meningitis experience long-term sequalae such as hearing loss, seizures, mental and motor abnormalities.
In each geographical region globally, PCV10 and PCV13 were shown to cover more than 70 percent of the serotypes causing IPD under 5 years of age during 1980–2007 prior to PCV introduction (PCV10 range 70–94 percent and 74–88 percent for PCV13).[8]
17.3.2. Global epidemiology since the introduction of pneumococcal conjugate vaccines
17.3.2. Global epidemiology since the introduction of pneumococcal conjugate vaccines
Direct impact of PCV programmes on IPD in children
Reductions in IPD among target cohorts of children in high income countries have been similar for PCV10 and PCV7/13 in reported studies. Québec (PCV10 and 13) and Finland (PCV10) both used 2+1 schedules and observed 83 percent and 79 percent reductions in IPD in vaccine-eligible children, respectively.[9, 10] In England, using PCV7 then PCV13 in a 2+1 schedule, there was an estimated 5,000 (54 percent) fewer hospital admissions for bacteraemia, meningitis and pneumonia in children aged under 5 years over 12 years after the introduction of PCV7 and PCV13. The greatest reductions were seen in meningitis (by 71 percent) in children under 2 years age.[11]
Direct impact of vaccination on non-invasive pneumococcal disease
The impact of pneumococcal conjugate vaccination on the large burden of non‑invasive pneumococcal disease has been clearly demonstrated internationally in countries that have introduced these vaccines, particularly through reductions in childhood hospitalisations due to pneumonia.[12, 13] Other impacts, such as on acute otitis media, are less clear and more difficult to measure accurately.[14] However, a systematic review found PCVs were associated with large reductions in risk of pneumococcal acute otitis media, but there was no evidence of benefit against all‑cause otitis media in high-risk children over 1 year of age or older children with a history of respiratory illness.[15]
Herd immunity
The extent to which childhood PCV immunisation programmes provide indirect reductions in IPD among high-risk children and older adults varies between reports, settings and vaccine serotype (notably serotypes 3 and 19A). There is some good evidence for the indirect (herd) effects of infant PCV immunisation on vaccine serotype pneumococcal disease in the non-vaccinated population, especially in adults aged 65 years and older, and an all age-effect on non-bacteraemic pneumonia.[16] This includes data showing reductions in the rates of IPD due to PCV7 and, more recently, PCV13 serotypes in non-vaccinated groups in many countries (for both pneumonia and IPD in adults) in North America and Europe.[11, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26] Reductions in adult pneumococcal pneumonia have also been observed in Western Kenya following the introduction of PCV10 in children[27] and in Japanese community-dwelling older adults following the introduction of PCV13.[28] These herd effects are predominantly due to decreased nasopharyngeal carriage of vaccine types in immunised children lowering transmission to unimmunised older children and adults.
Although many countries have reported significant decreases in vaccine-type IPD among children and the wider population following the introduction of PCVs to the childhood schedules, IPD due to non-PCV serotypes has increased in some, particularly in older adults.[29, 30] Therefore, for direct protection against a broad range of serotypes 23PPV continues to be necessary for those at highest risk of IPD.
17.3.3. New Zealand epidemiology
17.3.3. New Zealand epidemiology
Pneumococcal disease occurs throughout the year, but is more common in the autumn and winter months.[31, 32] Incidence of IPD is highest in infants and the elderly, especially among Māori and Pacific peoples.[31, 33, 34, 35]
Isolates from cases of IPD are serotyped at ESR and detailed information by age group is regularly updated on the ESR Public Health Surveillance website.
Incidence and mortality
In 2021, there were 468 notified IPD cases and the overall notification rate was 9.2 cases per 100,000 population (ESR, 3 May 2022). The highest rates of IPD were in adults aged 85 years and older (47.5 per 100,000) and in children aged under 1 year (46.0 per 100,000), followed by adults aged 75–84 years (24.3 per 100,000) and 65–74 years (17.8 per 100,000). The age-standardised rates of IPD were highest for the Pacific peoples (31.0 per 100,000, 64 cases) and Māori (26.5 per 100,000, 149 cases) ethnic groups. These rates were 4.9 and 4.2 times higher than the age-standardised rate for the European/Other ethnic group (6.3 per 100,000, 217 cases). The incidence of IPD was 12.5-fold higher for those living in areas with the highest levels of deprivation than those living in low deprivation areas across all age groups (60.9 vs 4.9 per 100,000). IPD was recorded as the primary cause of death for 16 cases in 2022, including 5 children aged under 5 years (the most since IPD became notifiable in late 2008). In 2021, the most reported risk factor in cases aged under 5 years were children who were born premature (7.6 percent) and children who were immunocompromised (4.5 percent) (ESR, 3 May 2022).
New Zealand epidemiology since the introduction of PCV
PCV7 was introduced in June 2008, PCV10 in July 2011 and PCV13 in July 2014, PCV10 replaced PCV13 in July 2017 on the routine Schedule (see Appendix 1). From October 2020, the number of primary doses of PCV10 were reduced to two (at age 6 weeks and 5 months) and the booster dose was brought forward from 15 months to 12 months. In December 2022, PCV13 replaced PCV10, given as a two-primary plus booster schedule routinely, and with an additional three-month dose of PCV13 for those with high-risk medical conditions as part of the extended immunisation programme.
IPD incidence
There have been dramatic reductions in the incidence of IPD in the vaccine-eligible age groups in New Zealand following the introduction of PCV to the Schedule in 2008 (see Figure 17.1).
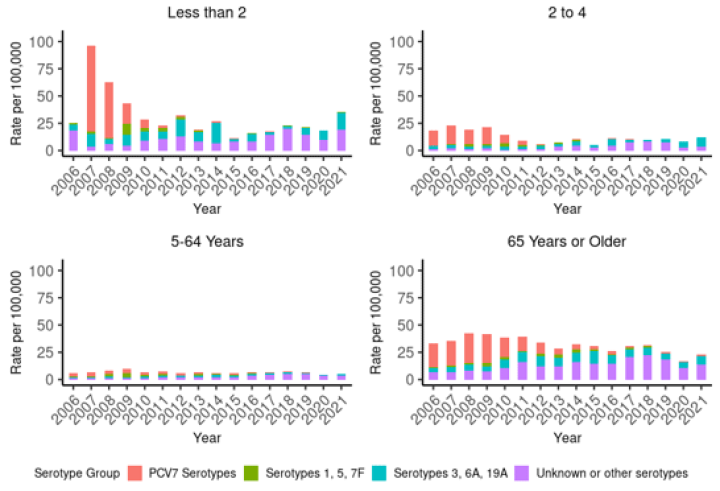
In children under 2 years of age, the total rate of IPD decreased by 74.3 percent since the introduction of PCV to the Schedule in late 2008: from 46.0 per 100,000 in 2009 to 11.8 per 100,000 in 2015. However, the rate has steadily increased since 2015 and in 2021 the rate of 35.7 per 100,000 was the highest since 2009.
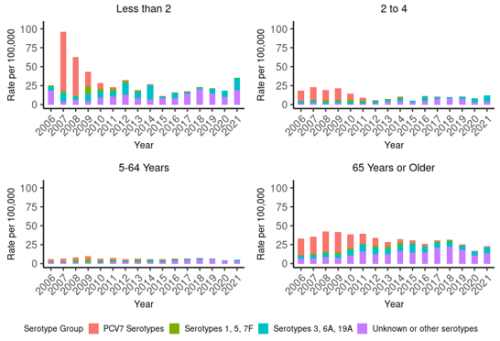
There has been a sustained reduction in PCV7 serotypes (see Figure 17.2), with few cases of PCV7 serotype IPD detected in children aged under 2 years since 2015 (ESR, 3 May 2022).
Similar reductions were seen for IPD caused by PCV10 serotypes under 2 years (see Figure 17.2). The incidence of all-cause IPD decreased in children aged 2–4 years from 2009 (by 45.9 percent; Figure 17.1), but have been rising again. Further, the rate of IPD due to PCV13-specific serotypes has been increasing since 2018 among children aged 2–4 years, though the rates are still lower than pre-2009 (Figure 17.2) (ESR, 3 May 2022). Serotype 19A has become an increasing problem, despite the theoretical cross-protection from 19F in the PCV10 vaccine.[36]
In 2021, 41.0 percent of cases in adults aged 65 years and over were PCV13 serotypes, and 73.8 percent were due to 23PPV serotypes. Of these, 37.0 percent (50/135 cases) were due to serotype 19A, and 44.4 percent were due to 23PPV-non-PCV13 serotypes (ESR, 3 May 2022).
Figure 17.1: Rate per 100,000 of invasive pneumococcal disease by age group and year, 2009–2021
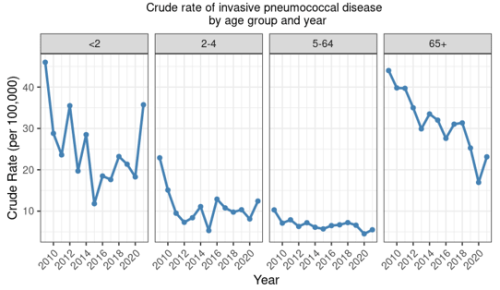
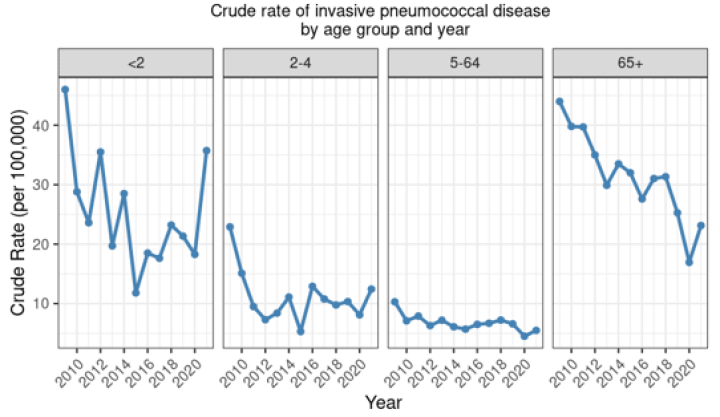
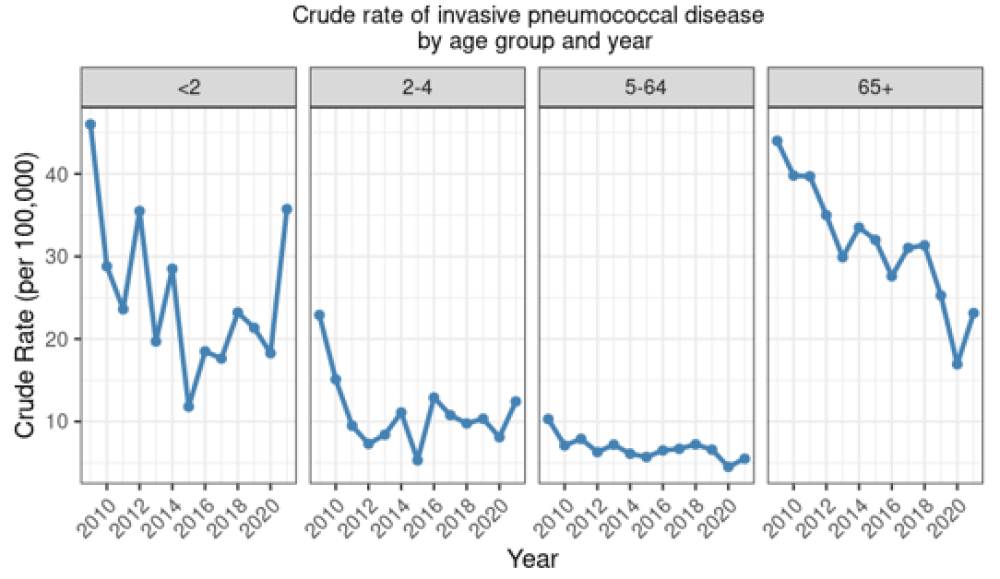
Figure 17.2: Rate per 100,000 population of invasive pneumococcal disease due to PCV7 serotypes, additional PCV10 types, additional PCV13 types and non-PCV types, by age group and year, 2006–2021
|
Notes: PCV7 was introduced in 2008, PCV10 in 2011, PCV13 in 2014 and PCV10 reintroduced in 2017. ‘PCV7 serotypes’ are cases due to serotypes covered by PCV7 (4, 6B, 9V, 14, 18C, 19F and 23F); ‘Serotypes 1, 5, 7F’ are cases due to the additional serotypes covered by PCV10; ‘Serotypes 3, 6A, 19A’ are cases due to the additional serotypes covered by PCV13; and ‘Unknown or Other serotypes’ are all other culture-positive IPD cases. IPD became a notifiable disease in 2008. Data presented from 2009 onwards is based on IPD notifications, and data prior to 2009 is from ESR’s national laboratory-based surveillance of IPD. |
Pneumococcal serotypes
Of the 468 IPD cases notified in 2021, 448 isolates were referred to ESR for serotyping. In children aged under 5 years, 49.2 percent (30/61) of cases were due to serotypes not covered by PCV, compared with 65.1 percent (136/209) and 57.9 percent (103/178) in the 5–64 years and 65 years and older age groups (ESR, 3 May 2022). In adults aged 65 years or older, serotype 23B and 16F were the most prevalent non-vaccine serotypes (PCV13 or 23PPV) in 2021 (ESR, 3 May 2022).
The number of IPD cases (246 cases) reported during July to September 2022 (Q3) was the highest of any year since IPD became a notifiable disease in 2008. In the 12 months to September 2022, the rate of 19A cases reached a record high of 23.3 cases per 100,000 in children aged under 2 years and 96 percent of PCV13-serotype cases in children under 5 years were 19A. The annualised rate was three times higher than the highest peak since IPD was notifiable: in children under 2 years the rate of 19A incidence is estimated to be 31 cases per 100,000 and 15 per 100,000 in 2–4-year-olds; and highest on record in those aged 65 years and older (10 cases per 100,000).[37]
Herd immunity
The addition of PCV to the New Zealand schedule in 2008 was followed by significant reductions in IPD due to PCV7 serotypes in age groups not eligible for routine infant immunisation (Figure 17.2). Since notification-based surveillance began in late 2008, the rate of IPD due to PCV7 serotypes in the 5–64-year age group decreased 96 percent from 3.8 per 100,000 to 0.2 per 100,000 in 2021, and the rate in cases aged 65 years and over decreased 94.3 percent from 26.6 to 1.5 per 100,000 (ESR, 3 May 2022). Though not as much of a decrease, the total rate of IPD in adults decreased by approximately 50 percent from 2009 to 2021 (rate for age 5–64 years: 10.3 to 5.5 per 100,000; age 65 years and over: 44.0 to 23.1 per 100,000.
Impact of vaccination on non-invasive pneumococcal disease
While hospitalisations for respiratory infections in children aged 5 years and under have been increasing in New Zealand, hospitalisations for all-cause pneumonia have declined significantly since the implementation of the pneumococcal conjugate vaccine programme in 2008. The largest reductions in all-cause pneumonia hospitalisations between 2006 and 2015 were in Māori (a 12 percent reduction) and Pacific children (a 21 percent reduction) and those living in areas of high deprivation.[38] A 51 percent decline in otitis media hospitalisations was observed for Māori children aged under 6 years following PCV immunisation, compared with 8 percent decline in otitis media across all ethnicities.[38]
Antimicrobial resistance
Introduction of pneumococcal conjugate vaccination reduced the circulation of resistant pneumococcal serotypes in the US,[39] but little change has been seen in New Zealand since PCV introduction. S. pneumoniae resistance to penicillin (14.1–23.5 percent) and cefotaxime resistance (0.4–2.1 percent) has varied year-to-year over the last decade with no significant trend.[40] A systematic review found that in countries with relatively high levels of pneumococcal antimicrobial resistance, the introduction of PCV13 was associated with reduction in antimicrobial resistance likely due to a reduction in serotype 19A.[41] However, heterogeneity between studies reflects the multiple drivers of antimicrobial resistance between countries and over time.[42]
In 2020, PCV7 serotypes accounted for 7 percent of the penicillin-resistant isolates compared with 92.8 percent in 2006/07, prior to vaccine introduction, but the prevalence of penicillin resistance among serotype 19A isolates increased just over four-fold from 15.8 percent in 2006/07 to 72.2 percent in 2020. The relative contribution of serotype 19A to penicillin-resistant invasive pneumococci had decreased, from a high of 52.1 percent in 2015 to 28.1 percent in 2019. In 2020, serotype 19A accounted for 44.8 percent of the penicillin-resistant isolates (ESR, 3 May 2022).
17.4. Vaccines
17.4.1. Available vaccines
17.4.1. Available vaccines
There are two types of pneumococcal vaccine approved for use in New Zealand for use against S. pneumoniae: pneumococcal conjugate vaccine (PCV) with ten or 13 serotypes and a plain polysaccharide pneumococcal vaccine (PPV) containing 23 serotypes. In PCVs, the pneumococcal surface polysaccharide is coupled to a carrier protein that induces increased production of type-specific antibodies, particularly in children aged under 2 years, and immunological memory enabling booster responses with subsequent doses (see section 1.4.3). Table 17.1 presents the polysaccharide serotypes contained within each vaccine.
Table 17.1: Pneumococcal vaccine serotype content

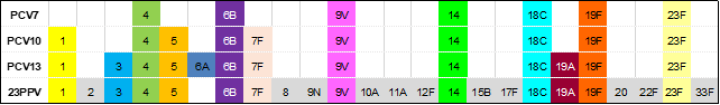
| Note: PCV10 contained serotype 6B and 19F, which elicit cross-reactive opsonophagocytic antibodies against serotype 6A and 19A, respectively, but at a lower level than PCV13.[8] The use of this vaccine has discontinued in New Zealand. |
Funded vaccines
PCV13 (Prevenar 13, Pfizer)
Each 0.5 mL dose of PCV13 contains:
- 2.2 μg of pneumococcal purified capsular polysaccharides for serotypes 1, 3, 4, 5, 6A, 7F, 9V, 14, 18C, 19A, 19F and 23F, and 4.4 μg of serotype 6B, conjugated to non-toxic diphtheria CRM197 protein and adsorbed onto aluminium phosphate (0.565 mg)
- succinic acid, polysorbate 80, aluminium phosphate, phosphate, and sodium chloride in water for injection.
23PPV (Pneumovax 23, MSD)
Each 0.5 mL dose of 23PPV contains:
- 25 µg of each capsular polysaccharide antigen (serotypes: 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33F)
- sodium chloride, water for injection, and phenol (0.25 percent) added as a preservative.
Other vaccine
A ten-valent pneumococcal conjugate vaccine, PCV10 (Synflorix, GSK), was used as part of the Schedule from 2017 to 2022, and previously from 2010 to 2014, with a 2+1 schedule (at ages 6 weeks, 5 months and 12 months) implemented routinely in 2020. This vaccine contains polysaccharides from pneumococcal serotypes 1, 4, 5, 6B, 7F, 9V, 14 and 23F conjugated to non-typeable Haemophilus influenzae (NTHi) protein D, serotype 18C conjugated to tetanus toxoid, and serotype 19F conjugated to diphtheria toxoid, adsorbed onto aluminium phosphate. The use of this vaccine was discontinued in New Zealand in December 2022.
17.4.2. Efficacy and effectiveness
17.4.2. Efficacy and effectiveness
13-valent pneumococcal conjugate vaccine
Childhood schedule
The immunogenicity of PCV13 in a 2+1 schedule (two primary plus booster) was found to be non-inferior to a PCV10 3+1 schedule (three primary doses plus booster) in Vietnam. In a clinical trial, 250 infants received PCV13 at ages 2, 4 and 9.5 months and were compared with 152 infants given PCV10 at ages 2, 3, 4 and 9.5 months or PCV10 as 2+1 schedule, and given concomitantly with DTaP-IPV-HepB/Hib. In the PCV13 group, more than 95 percent of infants responded to most serotypes after the primary doses (except for serotypes 6B and 23F). The magnitude of the response was greater for eight of the shared serotypes in the PCV13 group than the PCV10 groups. At 9 months (pre-booster), 75.4 percent to 100 percent of PCV10 groups and 68.9 percent to 99.1 percent of the PCV13 group had seroprotective antibody levels to most of the ten shared serotypes. Post-booster more than 97 percent of participants had IgG concentrations ≥0.35 µg/ml for all ten shared serotypes. Geometric mean concentrations followed a similar pattern post-booster to that seen post-primary for most serotypes. Only modest responses were seen against serotype 3 in the PCV13 group. For non-PCV10 serotypes, 94 percent of infants who received PCV13 had seroprotective levels of IgG against serotypes 3, 6A and 19A post primary and 99 percent post booster. At age 18 months, at least 95 percent of participants had seroprotective IgG levels for serotypes 14 and 19F, and for 6B (in PCV10 group), and more than 59 percent for all other shared serotypes.[43]
When breakthrough cases of IPD caused by serotype 19A were investigated in Québec, 19 of 31 cases (61 percent) occurred between ages 8 and 14 months, following two primary doses of PCV13 and prior to the toddler dose. Although PCV10-serotype 19F showed direct cross-protection against 19A in infants, there was no evidence of reduction in 19A carriage or indirect (herd) reductions in adults, whereas PCV13 did reduce 19A carriage and induce herd effects. It was proposed that a recent upswing in 19A may be due to a window of susceptibility in infants under a 2+1 schedule for PCV13 as 19A continues to circulate, but this was anticipated to decrease with stronger herd effects over time.[9]
Individuals at increased risk of IPD
Few studies have investigated the immunogenicity and effectiveness of PCV13 in individuals at increased risk of IPD. Studies using pneumococcal vaccines with similar but fewer antigens have demonstrated vaccine efficacy in individuals with immunocompromising conditions (eg, HIV, sickle cell disease), but the duration of protection against IPD remains unknown.[44] High IgG titres have been demonstrated following PCV13 vaccination of children with sickle cell disease,[45] HIV infection[46] and nephrotic syndrome.[47]
WHO recommends that children with medical conditions that reduce humoral immune response to vaccines, such as HIV, sickle cell disease and primary immune deficiencies, to have a 3+1 schedule of PCV13.[8] In children and adolescents with underlying medical conditions, such as type-1 diabetes, cancer, cystic fibrosis or asthma, the broader serotype protection provided by PCV13 can reduce nasopharyngeal carriage and the associated risk of IPD.[48, 49, 50, 51]
Use of pneumococcal conjugate vaccines in adults
PCV13 induces robust immune responses in adults,[52, 53, 54, 55] including elderly adults.[56] Although the antibody titres vary with serotype and between age groups, particularly for those aged over 65 years, the clinical significance of this variation is unclear.[53] PCV13 is at least as immunogenic as 23PPV in adults. Some studies suggest that prior 23PPV attenuates the immune response to PCV13, an effect not seen if PCV13 is given before 23PPV; conversely given first, PCV13 may augment the response to subsequent 23PPV vaccination.[56, 57, 58]
With respect to clinical outcomes, the Community-Acquired Pneumonia Immunization Trial in Adults (CAPITA) was a large, randomised, placebo-controlled trial conducted in the Netherlands that assessed efficacy of PCV13 against pneumococcal community-acquired pneumonia (CAP) with and without IPD in adults aged 65 years and older. The efficacy of PCV13 against vaccine-type IPD was 75 percent (95% CI: 41.4–90.8) and 45.6 percent (95% CI: 21.8–62.5) against vaccine-type pneumococcal CAP; 45.0 percent (95% CI: 14.2–65.3) for both combined.[59] Although this study showed individual protection for vaccine-type CAP, there was no significant reduction in all-cause pneumonia.[59]
An important uncertainty is the extent of indirect protection against vaccine-type IPD cases stemming from childhood immunisation programmes, which varies between countries. Where vaccine serotypes are sufficiently prevalent, PCV13 would provide some protection against all-cause CAP and lobular pneumonia.[60] Some of the non-PCV13 vaccine serotypes more likely to cause disease in adults are covered by 23PPV.
Data is limited for younger adults and specific at-risk adult populations.
23-valent vaccine pneumococcal polysaccharide
The polysaccharide vaccine (23PPV, Pneumovax 23) is made from the purified capsular polysaccharides of 23 serotypes of S. pneumoniae. It is available in New Zealand for adults and children from age 2 years. The 23 serotypes included in 23PPV (see Table 17.1) are responsible for about 90 percent or more of IPD in high-income countries.
See recent IPD surveillance reports from ESR for prevalence of serotypes covered by 23PPV in New Zealand (available on the Public Health Surveillance website).
A meta-analysis of IPD in adults aged from 65 years in 10 European countries showed that incidence of PCV7 serotypes had declined by 77 percent and for additional PCV13 serotypes by 38 percent after 5 years of PCV13/PCV10 immunisation programmes. The incidence rate of 23PPV-non-PCV13 serotypes had increased by around 50 percent with these 11 serotypes causing 22–54 percent of IPD.[61] In 2016, more than two-thirds of IPD cases in adults age 65 years or older were 23PPV-non-PCV13 serotypes.[40]
The efficacy of 23PPV varies depending on whether immune-competent or immunocompromised patients are studied, and whether the end point is pneumococcal pneumonia or bacteraemia.
The limitations of the polysaccharide vaccine have been summarised as:
- reduced efficacy in high-risk individuals
- uncertain efficacy against pneumonia
- it is only suitable for children aged 2 years and older.
- waning protection 2.5 to 5 years after vaccination.
A 2017 meta-analysis from Germany found pooled VE for 23PPV against any serotype IPD of 45 percent (95% CI: 15–65), 59 percent (95% CI: 35–74) or 73 percent (95% CI: 10–92) across cohort, case-control or clinical trial data; and pooled VE against any serotype pneumococcal pneumonia of 48 percent (95% CI: 25–63) and 64 percent (95% CI: 35–80) in cohort studies and clinical trials.[62] For both outcomes, waning of protection was found between 2.5 years and 5 years of follow-up after 23PPV.[62] Other systematic reviews, with differing eligibility criteria, found lower pooled VE estimates against IPD or pneumococcal pneumonia for 23PPV.[63, 64, 65] A Japanese prospective study found 23PPV to have moderate but variable effectiveness against vaccine-type pneumococcal pneumonia in adults aged 65 years or older.[66] Hence, questions remain around the clinical effectiveness and intervals between repeat doses of 23PPV that provide continued protection.
17.4.3. Dosage and administration
17.4.3. Dosage and administration
The dose of PCV13 and 23PPV is 0.5 mL, administered by intramuscular injection (see section 2.2.3). 23PPV can also be administered by subcutaneous injection (see section 2.2.3), but there is an increased likelihood of injection-site reactions.[67]
Co-administration with other vaccines
PCV13 or 23PPV may be administered at the same time as other routine childhood vaccinations, in a separate syringe at a separate injection site (see section 2.2.7 for information about multiple injections at the same visit).
PCV13 has been associated with increased risk of fever over 39°C and febrile convulsions when co-administered with inactivated influenza vaccine in children aged 6 months to under 5 years. Separation of the vaccines by two days can be offered but is not essential (see section 17.6.2). Systemic reactions have been noted in adults aged over 65 years.
17.4.4. Transport, storage and handling
17.4.4. Transport, storage and handling
Transport according to the National Standards for Vaccine Storage and Transportation for Immunisation Providers 2017 (2nd edition).
Store at +2°C to +8°C. Do not freeze.
17.5. Recommended immunisation schedule
17.5.1. Usual childhood schedule
17.5.1. Usual childhood schedule
Children aged under 5 years
PCV13 (Prevenar 13) vaccine is funded for all children aged under 5 years. Two doses of PCV13 are given as the primary course, with a booster at age 12 months (Table 17.2). Children who started their immunisation course with PCV10 can complete it with PCV13.
Table 17.2: Usual childhood PCV schedule
|
Age |
Vaccine |
Comment |
|---|---|---|
|
6 weeks |
PCV13 |
Primary series |
|
5 months |
PCV13 |
Primary series |
|
12 months |
PCV13 |
Booster |
Where a previously unimmunised child aged under 5 years presents late for pneumococcal vaccination, the age-appropriate catch-up schedules in Appendix 2 should be followed.
17.5.2. Extended pneumococcal immunisation for high-risk groups
17.5.2. Extended pneumococcal immunisation for high-risk groups
As part of the extended immunisation programme for high-risk groups, PCV13 and 23PPV are funded for eligible individuals, as shown in Table 17.3, Table 17.4 and Table 17.5. Because the recommended schedule depends on the age of the individual at diagnosis, the tables have been organised into age groups (under 5 years, 5–18 years and 18 years and older).
The PCV13 and 23PPV funding restrictions are as follows. See Table 17.3, Table 17.4 and Table 17.5 for the eligible conditions and dosing requirements.
PCV13
All high-risk infants (aged under 12 months) are recommended to receive three doses of a PCV vaccine, with at least one booster dose at or after 12 months of age. Change to the PCV13 extended schedule as soon as the infant is diagnosed as being at high risk (a dose is given at age 3 months in addition to the usual schedule).
- Two doses of PCV13 are funded for high-risk children aged from 12 months and under 18 years irrespective of previous doses of any non-PCV13 vaccine (PCV10 or PCV7); or,
- Up to an additional four doses of PCV13 are funded for vaccination or re-vaccination of high-risk children aged under 5 years.
- Up to an additional four doses of PCV13 are funded for vaccination or re-vaccination of eligible individuals aged 5 years and older.
23PPV
- Up to three doses of 23PPV are funded for individuals with eligible conditions.
- Up to two doses of 23PPV are funded for high-risk children aged under 18 years.
See also section 17.5.3 ‘(Re)vaccination’. See sections 4.2 and 4.3 for more information about immunocompromised infants, children and adults, including additional vaccine recommendations and schedule tables for certain conditions.
Table 17.3: Extended pneumococcal immunisation for children aged under 5 years – funded PCV13 and 23PPV indications and schedules
Table 17.3: Extended pneumococcal immunisation for children aged under 5 years – funded PCV13 and 23PPV indications and schedules
| See the Pharmaceutical Schedule for any changes to funding decisions. |
|
PCV13 (Prevenar 13) and 23PPV (Pneumovax 23) are funded for children aged under 5 years with any of the following:
|
||
|
Age at diagnosis |
Vaccine |
Recommended vaccine schedule |
|---|---|---|
|
<12 months |
PCV13 |
PCV13 at ages 6 weeks, 3, 5b and 12 months or an age-appropriate catch-up schedule. For those who have not been immunised at age 7–11 months – give 2 doses of PCV13 (8 weeks apart) and a further dose 8 weeks later, from age 12 months. For children aged 7–11 months who have completed a 2-dose primary course with PCV10, give 1 dose of PCV13 as soon as possible and another dose (of PCV13) 8 weeks later, from age 12 months. |
|
23PPV |
Following the completion of the PCV course, give 1 dose of 23PPV at age ≥2 years. There must be at least 8 weeks between the last PCV dose and the 23PPV dose. If risk persists, revaccinate once with 23PPV, 5 years after the first 23PPV. |
|
|
12 months to <5 years |
PCV13 |
For children who have not yet received any PCV13, give 2 doses of PCV13 at least 8 weeks apart.c,d |
|
23PPV |
Give 1 dose at least 8 weeks after the last PCV13 dose, from age 2 years. If risk persists, revaccinate once with 23PPV, 5 years after the first 23PPV. |
|
|
a. Children aged from 2 years who were born prematurely <28 weeks can benefit from 23PPV if they have ongoing lung disease b. A dose of PCV13 is given at 3 months, in addition to the usual Schedule. c. If 23PPV has already been given (prior to any doses of PCV13) to children aged under 5 years, wait at least 8 weeks before administering PCV13 (note: this timing differs in adults, see footnote in Table 17.5). d. There are no safety concerns, regardless of the interval between the last dose of PCV10 and the first dose of PCV13. |
||
Table 17.4: Extended pneumococcal immunisation for children aged from 5 to under 18 years – funded PCV13 and 23PPV indications and schedules
Table 17.4: Extended pneumococcal immunisation for children aged from 5 to under 18 years – funded PCV13 and 23PPV indications and schedules
|
PCV13 (Prevenar 13) and 23PPV (Pneumovax 23) are funded for children aged 5 to under 18 years:
Additionally, 23PPV (Pneumovax 23) is funded for children aged 5 to under 18 years:
|
||
|
Age at diagnosis |
Vaccine |
Recommended vaccine schedule |
|---|---|---|
|
5 years to <18 years |
PCV13 |
For children who have not previously received PCV13 |
|
23PPV |
1 dose of 23PPV at least 8 weeks after the PCV13 dose. If risk persists, revaccinate once with 23PPV, 5 years after the first 23PPV. |
|
|
a. PCV13 is funded pre- or post-HSCT or chemotherapy. 23PPV is only funded post-HSCT or chemotherapy. b. If 23PPV has already been given (prior to any doses of PCV13) to children aged under 18 years, wait at least 8 weeks before administering PCV13. c. There are no safety concerns, regardless of the interval between the last dose of PCV10 and the first dose of PCV13. d. See section 4.3.2 for children with Down syndrome. e. Children aged from 2 years who were born prematurely <28 weeks can benefit from 23PPV if they have ongoing lung disease. If lung disease persists, a further dose may be required to be given after 5 years. |
||
Table 17.5: Extended pneumococcal immunisation for adults aged 18 years and older – funded PCV13 and 23PPV indications and schedules
Table 17.5: Extended pneumococcal immunisation for adults aged 18 years and older – funded PCV13 and 23PPV indications and schedules
|
PCV13 (Prevenar 13) and 23PPV (Pneumovax 23) are funded for (re)vaccination of patients:
|
||
|
Age at diagnosis |
Vaccine |
Recommended vaccine schedule |
|---|---|---|
|
≥18 years |
PCV13 |
Give one dose of PCV13c |
|
23PPV |
Give a maximum of 3 doses of 23PPV in a lifetime, a minimum of 5 years apart. The first 23PPV dose is given at least 8 weeks after PCV13, the 2nd a minimum of 5 years later, and the 3rd dose at age ≥65 years. |
|
|
a. PCV13 is funded pre- or post-HSCT or chemotherapy. 23PPV is only funded post HSCT or chemotherapy. b. Only PCV13 is funded for these indications; 23PPV is recommended but not funded. c. If 23PPV has already been given (prior to any doses of PCV13) to adults aged 18 years and older, wait at least 1 year before administering PCV13. |
||
17.5.3. (Re)vaccination
17.5.3. (Re)vaccination
Up to an additional four doses of PCV13 and up to additional three doses of 23PPV are funded for vaccination or re vaccination of high-risk individuals (as listed in Table 17.3, Table 17.4 and Table 17.5).
See also sections 4.2.5 and 4.6.
17.5.4. Recommended but not funded
17.5.4. Recommended but not funded
Risk stacking
Two classifications of IPD risk are recognised: ‘high-risk’ conditions for which there is significant risk of IPD and ‘at‑risk’ conditions, which on their own may not significantly increase risk, but when combined or with added lifestyle risk factors increase an individual’s risk of IPD. This is described as ‘risk stacking’ – IPD incidence substantially increases with the accumulation of concurrent risk factors or conditions.[3, 4] The risk of pneumococcal infections in those with two or more at-risk conditions may be as high as the risk for those with a recognised high-risk condition.[68, 69, 70]
Recommendations
PCV13 and 23PPV are recommended but not funded for the following individuals:
- immune-competent adults (aged 18 years and older) at increased risk of pneumococcal disease or its complications because of chronic illness (eg, chronic heart, renal, liver or pulmonary disease, diabetes or alcohol dependency)
- immunocompromised adults at increased risk of pneumococcal disease (eg, those with nephrotic syndrome, multiple myeloma, lymphoma and Hodgkin’s disease)
- individuals of any age who have had one episode of IPD
- smokers.
PCV13 is funded but 23PPV is also recommended and not funded for individuals with:
- intracranial shunts
- cerebrospinal fluid leakage.
For those individuals who choose to purchase PCV13 and 23PPV vaccines, providers may follow the age-appropriate schedules in Table 17.4 and Table 17.5.
Adults aged 65 years and older with no other risk factors
Give one dose of PCV13 followed at least eight weeks later with 23PPV (not funded).
17.5.5. Pregnancy and breastfeeding
17.5.5. Pregnancy and breastfeeding
Pneumococcal vaccines are not routinely recommended for pregnant women.
Women of childbearing age who are eligible for funded PCV13 and 23PPV should be vaccinated before a planned pregnancy or as soon as possible after delivery (see Table 17.5). Administration of these vaccines in pregnancy is unlikely to result in serious adverse effects and may be considered in individuals at the very high risk of IPD who were not vaccinated prior to pregnancy.[71]
PCV13 and 23PPV may be given to breastfeeding women.[71]
17.6. Contraindications and precautions
See section 2.1.3 for pre-vaccination screening guidelines and section 2.1.4 for general contraindications for all vaccines.
17.6.1. Contraindications
17.6.1. Contraindications
There are no specific contraindications to pneumococcal polysaccharide or conjugate vaccines apart from a severe reaction to a previous dose or known hypersensitivity to any components of either vaccine.
17.6.2. Precautions
17.6.2. Precautions
Systemic reactions (chills, rash and myalgia) may occur when PCV13 and influenza vaccine are administered at the same time. PCV13 has been associated with a slightly higher risk of fever over 39°C and febrile convulsions when co-administered with inactivated influenza vaccine in infants and young children, compared to when administered separately.[72] Febrile convulsion history is not a contraindication to PCV13 immunisation. If indicated, PCV13 and influenza vaccines may be given to a child aged under 5 years at the same visit.[71] Parents/guardians should be informed of the small risk of febrile convulsions, and separation of vaccines by two days can be offered. If the child has a history of febrile convulsions, separation of the vaccines is recommended.
23PPV should not be given to children aged under 2 years due to the reduced immune response associated with polysaccharide vaccines (see section 1.4.3).
17.7. Potential responses and AEFIs
17.7.1. Pneumococcal conjugate vaccines
17.7.1. Pneumococcal conjugate vaccines
Pneumococcal conjugate vaccines have excellent safety profiles. A 2016 systematic review found that pneumococcal conjugate vaccines are considered safe for use in children, and serious adverse events are detected very rarely by post-marketing surveillance.[73]
The most commonly reported adverse reactions after PCV13 are injection-site reactions, fever, irritability, decreased appetite and increased or decreased sleep.[74] An increase in injection-site reactions was reported in children older than 12 months compared to rates observed in infants during the primary series with PCV13.
No serious adverse events have been identified in adults or children, associated with underlying disease or immunocompromise.[75, 76, 77]
17.7.2. Pneumococcal polysaccharide vaccine
17.7.2. Pneumococcal polysaccharide vaccine
Local discomfort, erythema and induration lasting a couple of days are potential responses.[78] Local and systemic reactions, such as self-limiting mild fever, myalgia and decreased arm movement in injected limb, may occur after revaccination of adults, particularly when the second dose is given within five years of the first dose.[71]
17.8. Public health measures
IPD is a notifiable condition, and if confirmed, the laboratory undertaking the testing must notify the local medical officer of health.
Local public health action is not expected in response to individual notifications of this disease. Passive surveillance for IPD and pneumococcal serotypes help to inform the immunisation schedule.
Antimicrobial prophylaxis is not indicated for close contacts of cases of IPD. For those at high risk of pneumococcal disease where response to vaccination may be poor, antimicrobial prophylaxis may be indicated. Discuss with an appropriate specialist.
For more details on control measures, refer to the ‘Invasive pneumococcal disease’ chapter of the Communicable Disease Control Manual.
17.9. Variations from the vaccine data sheet
The PCV13 (Prevenar 13) data sheet states that there is no data on the interchangeability of PCV13 with other pneumococcal conjugate vaccines containing a protein carrier different from CRM197. Health NZ | Te Whatu Ora of Health recommends that those who started with PCV10 may complete with PCV13 (see section 17.5).
Children aged 24–59 months who have not received any PCV, or only one dose of PCV10 before the age of 12 months, are recommended to receive two doses of PCV13 given 8 weeks apart rather than one dose as given on the PCV13 (Prevenar 13) datasheet (see Appendix 2, Table A2.6).
References
References
References
- American Academy of Pediatrics. 2021. Pneumococcal infections. in Red Book: 2021 Report of the Committee on Infectious Diseases, Kimberlin D, Brady M, Jackson M, et al. (eds). Elk Grove Village, IL. p. 639-650. URL: https://redbook.solutions.aap.org/redbook.aspx. (accessed 3 Nov 2022)
- World Health Organization. Pneumococcal vaccines – WHO position paper, 2012. Weekly Epidemiological Record, 2012. 87(14): p. 129–44.
- Shea KM, Edelsberg J, Weycker D, et al. Rates of pneumococcal disease in adults with chronic medical conditions. Open Forum Infect Dis, 2014. 1(1): p. ofu024.
- Morton JB, Morrill HJ, LaPlante KL, et al. Risk stacking of pneumococcal vaccination indications increases mortality in unvaccinated adults with Streptococcus pneumoniae infections. Vaccine, 2017. 35(13): p. 1692-1697.
- Dirmesropian S, Liu B, Wood JG, et al. Pneumonia hospitalisation and case-fatality rates in older Australians with and without risk factors for pneumococcal disease: implications for vaccine policy. Epidemiology and Infection, 2019. 147: p. e118.
- Seminog OO ,Goldacre MJ. Risk of pneumonia and pneumococcal disease in people with severe mental illness: English record linkage studies. Thorax, 2013. 68(2): p. 171-176.
- Chapman KE, Wilson D ,Gorton R. Invasive pneumococcal disease and socioeconomic deprivation: A population study from the North East of England. Journal of Public Health (United Kingdom), 2013. 35(4): p. 558-569.
- World Health Organization. Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper – February 2019. Weekly Epidemiological Record, 2019. 94(8): p. 85-104.
- De Wals P, Lefebvre B, Deceuninck G, et al. Incidence of invasive pneumococcal disease before and during an era of use of three different pneumococcal conjugate vaccines in Quebec. Vaccine, 2018. 36(3): p. 421-426.
- Rinta-Kokko H, Palmu AA, Auranen K, et al. Long-term impact of 10-valent pneumococcal conjugate vaccination on invasive pneumococcal disease among children in Finland. Vaccine, 2018. 36(15): p. 1934-1940.
- Shiri T, McCarthy ND ,Petrou S. The impact of childhood pneumococcal vaccination on hospital admissions in England: a whole population observational study. BMC Infectious Diseases, 2019. 19(1): p. 510.
- Lucero MG, Dulalia VE, Nillos LT, et al. Pneumococcal conjugate vaccines for preventing vaccine-type invasive pneumococcal disease and X-ray defined pneumonia in children less than two years of age. Cochrane Database Syst Rev, 2009(4): p. CD004977.
- Fitzwater SP, Chandran A, Santosham M, et al. The worldwide impact of the seven-valent pneumococcal conjugate vaccine. Pediatric Infectious Disease Journal, 2012. 31(5): p. 501-8.
- Taylor S, Marchisio P, Vergison A, et al. Impact of pneumococcal conjugate vaccination on otitis media: a systematic review. Clinical Infectious Diseases, 2012. 54(12): p. 1765-73.
- Fortanier AC, Venekamp RP, Boonacker CW, et al. Pneumococcal conjugate vaccines for preventing acute otitis media in children. Cochrane Database Syst Rev, 2019. 5: p. CD001480.
- Simonsen L, Taylor RJ, Young-Xu Y, et al. Impact of pneumococcal conjugate vaccination of infants on pneumonia and influenza hospitalization and mortality in all age groups in the United States. MBio, 2011. 2(1): p. e00309-10.
- Ahmed SS, Pondo T, Xing W, et al. Early impact of 13-valent pneumococcal conjugate vaccine use on invasive pneumococcal disease among adults with and without underlying medical conditions – United States. Clinical Infectious Diseases, 2019.
- Centers for Disease Control and Prevention. Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease – United States, 1998–2003. Morbidity and Mortality Weekly Report, 2005. 54(36): p. 893–7.
- Demczuk WH, Martin I, Griffith A, et al. Serotype distribution of invasive Streptococcus pneumoniae in Canada after the introduction of the 13-valent pneumococcal conjugate vaccine, 2010–2012. Canadian Journal of Microbiology, 2013. 59(12): p. 778-88.
- Elberse KE, van der Heide HG, Witteveen S, et al. Changes in the composition of the pneumococcal population and in IPD incidence in The Netherlands after the implementation of the 7-valent pneumococcal conjugate vaccine. Vaccine, 2012. 30(52): p. 7644-51.
- Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. New England Journal of Medicine, 2013. 369(2): p. 155-63.
- Ingels H, Rasmussen J, Andersen PH, et al. Impact of pneumococcal vaccination in Denmark during the first 3 years after PCV introduction in the childhood immunization programme. Vaccine, 2012. 30(26): p. 3944-50.
- Miller E, Andrews NJ, Waight PA, et al. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infectious Diseases, 2011. 11(10): p. 760-8.
- Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. Journal of Infectious Diseases, 2010. 201(1): p. 32-41.
- Steens A, Bergsaker MA, Aaberge IS, et al. Prompt effect of replacing the 7-valent pneumococcal conjugate vaccine with the 13-valent vaccine on the epidemiology of invasive pneumococcal disease in Norway. Vaccine, 2013. 31(52): p. 6232-8.
- Vestrheim DF, Hoiby EA, Bergsaker MR, et al. Indirect effect of conjugate pneumococcal vaccination in a 2+1 dose schedule. Vaccine, 2010. 28(10): p. 2214-2221.
- Bigogo GM, Audi A, Auko J, et al. Indirect effects of 10-valent pneumococcal conjugate vaccine against adult pneumococcal pneumonia in rural Western Kenya. Clinical Infectious Diseases, 2019.
- Sando E, Suzuki M, Furumoto A, et al. Impact of the pediatric 13-valent pneumococcal conjugate vaccine on serotype distribution and clinical characteristics of pneumococcal pneumonia in adults: The Japan Pneumococcal Vaccine Effectiveness Study (J-PAVE). Vaccine, 2019. 37(20): p. 2687-2693.
- Naucler P, Galanis I, Morfeldt E, et al. Comparison of the Impact of Pneumococcal Conjugate Vaccine 10 or Pneumococcal Conjugate Vaccine 13 on Invasive Pneumococcal Disease in Equivalent Populations. Clinical Infectious Diseases, 2017. 65(11): p. 1780-1789.
- Levy C, Varon E, Ouldali N, et al. Changes in invasive pneumococcal disease spectrum after 13 valent pneumococcal conjugate vaccine implementation. Clinical Infectious Diseases, 2019.
- Singh KP, Voolmann T ,Lang SD. Pneumococcal bacteraemia in south Auckland: a five year review with emphasis on prescribing practices. New Zealand Medical Journal, 1992. 105(943): p. 394-5.
- Murdoch DR ,Jennings LC. Association of respiratory virus activity and environmental factors with the incidence of invasive pneumococcal disease. Journal of Infection, 2009. 58(1): p. 37-46.
- Voss L, Lennon D, Okesene-Gafa K, et al. Invasive pneumococcal disease in a pediatric population, Auckland, New Zealand. Pediatric Infectious Disease Journal, 1994. 13(10): p. 873-8.
- Chambers S, Laing R, Murdoch D, et al. Maori have a much higher incidence of community-acquired pneumonia and pneumococcal pneumonia than non-Maori: findings from two New Zealand hospitals. New Zealand Medical Journal, 2006. 119(1234): p. U1978.
- Heffernan HM, Martin DR, Woodhouse RE, et al. Invasive pneumococcal disease in New Zealand 1998-2005: capsular serotypes and antimicrobial resistance. Epidemiology and Infection, 2008. 136(3): p. 352-9.
- Anglemyer A, McNeill A, DuBray K, et al. Invasive Pneumococcal Disease: Concerning Trends in Serotype 19A Notifications in New Zealand. Clinical Infectious Diseases, 2022. 74(10): p. 1859-1861.
- Institute of Environmental Science and Research. 2022 Invasive pneumococcal disease quarterly report, July-September 2022. Poirua. URL: https://surv.esr.cri.nz/surveillance/IPD.php?we_objectID=5257. (accessed 13 December 2022)
- Petousis-Harris H, Howe AS, Paynter J, et al. Pneumococcal conjugate vaccines turning the tide on inequity: a retrospective cohort study of New Zealand children born 2006–2015. Clinical Infectious Diseases, 2019. 68(5): p. 818-826.
- Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. New England Journal of Medicine, 2006. 354(14): p. 1455-63.
- Institute of Environmental Science and Research Ltd (ESR). 2019. Invasive pneumococcal disease in New Zealand, 2016 (ed.), Porirua: ESR. URL: https://surv.esr.cri.nz/PDF_surveillance/IPD/2016/2016IPDAnnualReport.pdf (accessed 25 November 2019)
- Tin Tin Htar M, van Den Biggelaar AHJ, Sings H, et al. The impact of routine childhood immunization with higher-valent pneumococcal conjugate vaccines on antimicrobial-resistant pneumococcal diseases and carriage: a systematic literature review. Expert Rev Vaccines, 2019. 18(10): p. 1069-1089.
- Hume-Nixon M, Lim R, Russell F, et al. Systematic review of the clinical outcomes of pneumonia with a penicillin-group resistant pneumococcus in respiratory and blood culture specimens in children in low- and middle-income countries. J Glob Health, 2022. 12: p. 10004.
- Temple B, Toan NT, Dai VTT, et al. Immunogenicity and reactogenicity of ten-valent versus 13-valent pneumococcal conjugate vaccines among infants in Ho Chi Minh City, Vietnam: a randomised controlled trial. The Lancet Infectious Diseases, 2019. 19(5): p. 497-509.
- Centers for Disease Control and Prevention. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among children aged 6–18 years with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report, 2013. 62(25): p. 521–4.
- Plosker GL. 13-valent pneumococcal conjugate vaccine: a review of its use in infants, children, and adolescents. Paediatric Drugs, 2013. 15(5): p. 403-23.
- Bhorat AE, Madhi SA, Laudat F, et al. Immunogenicity and safety of the 13-valent pneumococcal conjugate vaccine in HIV-infected individuals naive to pneumococcal vaccination. AIDS, 2015. 29(11): p. 1345-54.
- Pittet LF, Posfay-Barbe KM, Chehade H, et al. Optimizing seroprotection against pneumococcus in children with nephrotic syndrome using the 13-valent pneumococcal conjugate vaccine. Vaccine, 2016. 34(41): p. 4948-4954.
- Esposito S, Terranova L, Patria MF, et al. Streptococcus pneumoniae colonisation in children and adolescents with asthma: impact of the heptavalent pneumococcal conjugate vaccine and evaluation of potential effect of thirteen-valent pneumococcal conjugate vaccine. BMC Infectious Diseases, 2016. 16(1): p. 12.
- Esposito S, Colombo C, Tosco A, et al. Streptococcus pneumoniae oropharyngeal colonization in children and adolescents with cystic fibrosis. J Cyst Fibros, 2016. 15(3): p. 366-71.
- Principi N, Iughetti L, Cappa M, et al. Streptococcus pneumoniae oropharyngeal colonization in school-age children and adolescents with type 1 diabetes mellitus: Impact of the heptavalent pneumococcal conjugate vaccine. Human Vaccines & Immunotherapeutics, 2016. 12(2): p. 293-300.
- Principi N, Preti V, Gaspari S, et al. Streptococcus pneumoniae pharyngeal colonization in school-age children and adolescents with cancer. Human Vaccines & Immunotherapeutics, 2016. 12(2): p. 301-7.
- Jackson LA, Gurtman A, van Cleeff M, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults. Vaccine, 2013. 31(35): p. 3577-84.
- Shiramoto M, Irie S, Juergens C, et al. Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine when administered to healthy Japanese adults aged >/=50 years. An open-label trial. Human Vaccines & Immunotherapeutics, 2014. 10(7): p. 1850-8.
- Bryant KA, Frenck R, Gurtman A, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 18-49 years of age, naive to 23-valent pneumococcal polysaccharide vaccine. Vaccine, 2015. 33(43): p. 5854-5860.
- Tinoco JC, Juergens C, Ruiz Palacios GM, et al. Open-label trial of immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults >/= 50 years of age in Mexico. Clinical and Vaccine Immunology, 2015. 22(2): p. 185-92.
- Jackson LA, Gurtman A, Rice K, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine, 2013. 31(35): p. 3585-93.
- Jackson LA, Gurtman A, van Cleeff M, et al. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine, 2013. 31(35): p. 3594-602.
- Plosker GL. 13-Valent Pneumococcal Conjugate Vaccine: A Review of Its Use in Adults. Drugs, 2015. 75(13): p. 1535-46.
- Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. New England Journal of Medicine, 2015. 372(12): p. 1114-25.
- Leesa F ,Spiller MT, Effectiveness of PCV13 in Adults Hospitalized with Pneumonia Using Centers for Medicare & Medicaid Services Data, 2014–2017 in ACIP meeting February 2019. 2019, National Center for Immunization & Respiratory Diseases,.
- Hanquet G, Krizova P, Valentiner-Branth P, et al. Effect of childhood pneumococcal conjugate vaccination on invasive disease in older adults of 10 European countries: implications for adult vaccination. Thorax, 2019. 74(5): p. 473-482.
- Falkenhorst G, Remschmidt C, Harder T, et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine (PPV23) against pneumococcal disease in the elderly: systematic review and meta-analysis. PloS One, 2017. 12(1): p. e0169368.
- Huss A, Scott P, Stuck AE, et al. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ: Canadian Medical Association Journal, 2009. 180(1): p. 48-58.
- Vila-Corcoles A, Ochoa-Gondar O, Guzman JA, et al. Effectiveness of the 23-valent polysaccharide pneumococcal vaccine against invasive pneumococcal disease in people 60 years or older. BMC Infectious Diseases, 2010. 10(73): p. 73.
- Cadeddu C, De Waure C ,Gualano MR. 23-valent pneumococcal polysaccharide vaccine (PPV23) for the prevention of invasive pneumococcal diseases (IPDs) in the elderly: is it really effective? Journal of Preventive Medicine and Hygiene, 2012. 53(2): p. 101–103.
- Suzuki M, Dhoubhadel BG, Ishifuji T, et al. Serotype-specific effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumococcal pneumonia in adults aged 65 years or older: a multicentre, prospective, test-negative design study. Lancet Infectious Diseases, 2017. 17(3): p. 313-321.
- Cook IF, Pond D ,Hartel G. Comparative reactogenicity and immunogenicity of 23 valent pneumococcal vaccine administered by intramuscular or subcutaneous injection in elderly adults. Vaccine, 2007. 25(25): p. 4767-74.
- Curcio D, Cané A ,Isturiz R. Redefining risk categories for pneumococcal disease in adults: critical analysis of the evidence. International Journal of Infectious Diseases, 2015. 37: p. 30-5.
- Pelton SI, Shea KM, Weycker D, et al. Rethinking risk for pneumococcal disease in adults: the role of risk stacking. Open Forum Infect Dis, 2015. 2(1): p. ofv020.
- Baxter R, Yee A, Aukes L, et al. Risk of underlying chronic medical conditions for invasive pneumococcal disease in adults. Vaccine, 2016. 34(36): p. 4293-7.
- Australian Technical Advisory Group on Immunisation (ATAGI). 2018. Pneumococcal disease. in Australian Immunisation Handbook. Canberra. URL: https://immunisationhandbook.health.gov.au/vaccine-preventable-diseases/pneumococcal-disease. (accessed 25 April 2020)
- Tse A, Tseng HF, Greene SK, et al. Signal identification and evaluation for risk of febrile seizures in children following trivalent inactivated influenza vaccine in the Vaccine Safety Datalink Project, 2010-2011. Vaccine, 2012. 30(11): p. 2024-31.
- Esposito S ,Principi N. Safety and tolerability of pneumococcal vaccines in children. Expert Opin Drug Saf, 2016. 15(6): p. 777-85.
- Thompson A, Gurtman A, Patterson S, et al. Safety of 13-valent pneumococcal conjugate vaccine in infants and children: meta-analysis of 13 clinical trials in 9 countries. Vaccine, 2013. 31(45): p. 5289-95.
- Ho YL, Brandao AP, de Cunto Brandileone MC, et al. Immunogenicity and safety of pneumococcal conjugate polysaccharide and free polysaccharide vaccines alone or combined in HIV-infected adults in Brazil. Vaccine, 2013. 31(37): p. 4047-53.
- Cordonnier C, Ljungman P, Juergens C, et al. Immunogenicity, safety, and tolerability of 13-valent pneumococcal conjugate vaccine followed by 23-valent pneumococcal polysaccharide vaccine in recipients of allogeneic hematopoietic stem cell transplant aged >/=2 years: an open-label study. Clinical Infectious Diseases, 2015. 61(3): p. 313-23.
- Glesby MJ, Watson W, Brinson C, et al. Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine in HIV-infected adults previously vaccinated with pneumococcal polysaccharide vaccine. Journal of Infectious Diseases, 2015. 212(1): p. 18-27.
- Bentley D, Ita K ,Moon D. Pneumococcal vaccine in the institutional elderly: design of a non-randomized trial and preliminary results. Reviews of Infectious Diseases, 1981. 3(Suppl): p. 571.