On this page
Key information
|
Mode of transmission |
By respiratory droplets or direct contact with nasopharyngeal secretions from a carrier or case. |
|---|---|
|
Incubation period |
2–10 days, commonly 3–4 days. |
|
Period of communicability |
Commonly 3–4 days without treatment, range 2–10 days. Certain antibiotic therapy eradicates N. meningitidis from mucosal surfaces within 24 hours, and the case is no longer considered infectious. |
|
Funded vaccines |
|
|
Dose, presentation, route |
0.5 mL per dose. Presentation:
Intramuscular injection. |
|
Funded vaccine indications |
MenB (Bexsero)
MenB (Bexsero) and MenACWY (Nimenrix, from age 6 weeks to under 12 months; MenQuadfi from age 12 months) for:
MenB and MenACWY (MenQuadfi) for:
|
|
Recommended, unfunded |
Laboratory workers handling bacterial cultures Health care professionals in very close contact with cases. |
|
Vaccine effectiveness |
MenB: 75% reduction in group B cases in infants over a 3-year period in the UK and cross-protection against group W observed; 71% reduction in group B disease in adolescents. MenACWY: 80–85%; effectiveness wanes to 50–60% within 2–5 years after vaccination. MenC: effectiveness of 83–100%; antibody wanes within 2–3 years. |
|
Potential responses to vaccines |
MenB: increased risk of fever and fever-related events in children <2 years (prophylaxic antipyretic advised). Older age groups: localised pain, nausea, myalgia, malaise, mild fever and headache. MenC and MenACWY: localised pain, irritability, headache and fatigue, mild fever. |
|
Contraindications |
No specific contraindications or precautions, except prior anaphylaxis to vaccine components. |
|
Public health measures |
All cases must be notified if clinically suspected. Parenteral antibiotics should be administered as soon as possible in hospital or before admission to hospital if delays of longer than 30 minutes are likely. |
|
Post-exposure prophylaxis |
For vaccination of contacts see section 13.8. |
13.1. Bacteriology
Meningococcal disease is caused by Neisseria meningitidis, a gram-negative bacterium, causing sepsis, meningitis and some less common clinical syndromes. Groups B and W are currently the most important types in New Zealand. Increasingly, group W and Y organisms are the cause of bacteraemia and pneumonia in the elderly. Predominant groups differ between countries; group A is an important epidemic strain, particularly in Africa and the Middle East. Meningococci are spread from person to person by respiratory droplets or direct contact with nasopharyngeal secretions from a carrier or case.
13.2. Clinical features
Table 13.1 below describes the symptoms and signs of meningococcal disease – individuals may present with some or all of these. Meningococcal septicaemia is more common than meningitis, and presentation varies from a mild non-specific illness to rapid progression with fatal outcome. Symptoms and signs in infants are frequently non-specific. The classical rapidly progressing petechial or purpuric rash may not be present or may initially appear maculopapular. Atypical initial presentations, including gastrointestinal symptoms, septic arthritis and epiglottitis, are more frequently reported with meningococcal W disease, and may contribute to delayed diagnosis and increased case-fatality.[1, 2] Pneumonia is more frequently reported with group Y.
Table 13.1: Symptoms and signs of meningococcal disease
Table 13.1: Symptoms and signs of meningococcal disease
Meningococcal disease covers a spectrum, from persistent fever with or without rash and arthritis to rapidly progressive purpuric rash and shock. Meningitis can occur with and without signs of sepsis. In fulminant cases, coma and death can occur within a few hours despite appropriate treatment.
Because of the potential for rapid progression, antibiotics should be administered (Table 13.2) as soon as possible, even before hospital admission. Antibiotics given prior to transfer should be clearly noted on information accompanying the patient to hospital.
Table 13.2: Recommended antibiotics for suspected cases
Table 13.2: Recommended antibiotics for suspected cases
| Check The New Zealand Formulary for up to date dosing details, as well as the Starship guidelines. |
|
Antibiotic |
Children <30kg |
Children >30kg and |
|---|---|---|
|
Ceftriaxonea |
50 mg/kg when given by GP/primary care 100 mg/kg IV (or IM) up to 2g when given in ED |
2 g IV (or IM) |
|
Benzylpenicillinb |
50 mg/kg IV (or IM) |
2.4 g IV (or IM) |
|
a. Patients allergic to penicillin who do not have a documented history of anaphylaxis to penicillin can be given ceftriaxone. b. Patients with a documented history of anaphylaxis to penicillin and who are suspected of suffering from meningococcal disease should be sent immediately to hospital without pre‑admission antibiotics. |
||
13.3. Epidemiology
13.3.1. Global burden of disease
13.3.1. Global burden of disease
Incidence and serotypes
The prevalence of meningococcal groups varies geographically. The highest burden of disease occurs in sub-Saharan Africa, where despite a dramatic fall in Group A disease following introduction of a Group A conjugate vaccine, in this ‘meningitis belt’ epidemics continue with around 30,000 cases reported annually, now including Group W.
The incidence in Canada, the US and Europe varies substantially from 0.2 to 3 per 100,000 persons per year.[3] Group B has become the predominant capsular group in Europe, Americas and Australia, with incidence typically highest in children aged under 2 years.[4] Group C disease has almost disappeared in countries with universal immunisation programmes, but outbreaks have been observed in men who have sex with men in the US and Europe.
Since 2009 there has been an emerging global incidence of Group W disease, initially in the United Kingdom and South America. Australia has experienced a rapid increase in Group W cases since 2013 with New Zealand also seeing a rapid increase in cases since 2017. Like group C clonal complex ST11 strains, group W ST11 strains have enhanced virulence. Higher rates of carriage of these ST11 strains has been noted within age groups where invasive group W disease is more prevalent (infants and the elderly).[5]
Some parts of the world, particularly Scandinavia, have reported an increase in group Y disease. In other regions, there is evidence of colonisation, but disease caused by group Y is rare. Patients with group Y strain disease are more likely to develop pneumonia and to be elderly.[3, 4]
This emergence of group W and Y strains has led to meningococcal C vaccines being replaced by quadrivalent (group A, C, W, Y) meningococcal conjugate vaccines (MenACWY).
Risk groups
The highest incidence of meningococcal disease occurs in children aged under 5 years (especially under 2 years) with a secondary peak in older adolescents (15–19 years).The age distribution for groups W and Y is more likely to include older people that for B and C. A pooled overall case-fatality rate of 8.3 percent (range 4.1–20 percent) is reported internationally, varying by group and age.[6]
Most infection occurs in healthy people, but those with certain rare immune deficiencies (of terminal components of complement (C5–9) or properdin) or asplenia are at much higher risk, particularly of recurrent meningococcal disease. Individuals with infection caused by groups other than A, B, C, W, Y and untypeable strains or who experience recurrent disease should be investigated.
Close contacts of primary cases of meningococcal infection are at increased risk of developing infection, such as the case’s household,[7] early childhood education services, semi-closed communities, schools, correctional facilities and military recruit camps. Students living in hostel accommodation may also be at higher risk.[8, 9, 10] In health care settings, only those with close exposure to oropharyngeal secretions of patients with meningococcal disease (as may occur during intubation or resuscitation) and microbiology laboratory workers are considered to be at increased risk.
It is not possible to calculate the incubation period for meningococcal disease for sporadic cases. Secondary cases (ie, in contacts of known cases of meningococcal disease) usually occur within four days, but it can be up to 10 days. The infectivity of patients with meningococcal disease is markedly reduced after 24 hours of antibiotic therapy, although treatment with cefotaxime, ceftriaxone, rifampicin or ciprofloxacin is necessary to reliably eradicate nasopharyngeal carriage.
In high-income countries in the absence of immunisation, nasopharyngeal carriage of N. meningitidis occurs in approximately 10 percent of the overall population, rising from 2 percent in children aged under 4 years to a peak of 24.5 percent to 32 percent among 15–24-year-olds, then declining with increasing age.[3, 11] In adolescents and young adults, the overall and capsular group carriage vary between regions and age groups.[12] The relationship between risk factors for disease and those associated with carriage is incompletely understood.[3] Carriage prevalence does not predict the disease incidence nor the occurrence or severity of outbreaks, as most of the carried strains are non-encapsulated and do not cause disease.[3] Smoking, passive smoking, household crowding and upper respiratory tract infections increase carriage.
13.3.2. New Zealand epidemiology
13.3.2. New Zealand epidemiology
Incidence and mortality
From 1 January to 31 December 2023, there were 59 cases of meningococcal disease (52 confirmed cases), which was an increase from the same period in 2020 and 2021 but lower than in 2017, 2018, 2019 and 2022. Of the 15 cases aged under 5 years, 87 percent were of Māori and/or Pacific ethnicity. There was one death in an adult aged 20–29 years.[13]
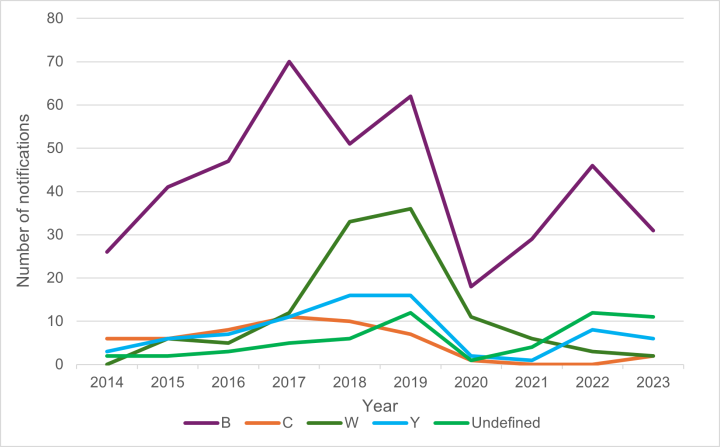
For 2023, the notification rate for meningococcal disease was 1.1 cases per 100,000 population, with a total of 59 cases notified (52 laboratory confirmed; ESR, 12 July 2024). Cases remained significantly lower than the peak annual incidence rate of 16.7 per 100,000 for all ages and 200 per 100,000 in children under 12 months as experienced in 2001 during the meningococcal epidemic from 1991 to 2007. The epidemic was largely due to a single group B subtype (B:P1.7-2,4). The annual number of notified cases of meningococcal disease in New Zealand since 1970 is shown in Figure 13.1.[13]
For further details and reports of meningococcal disease in New Zealand refer to the ESR surveillance reports.
Figure 13.1: Notified cases of meningococcal disease, 1970–2023
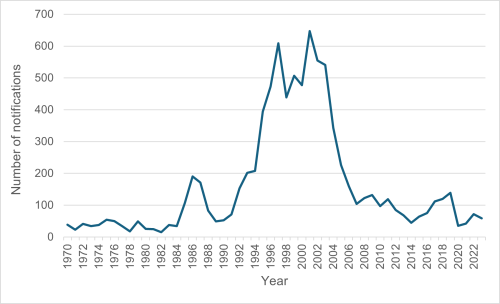
Source: ESR
Meningococcal disease incidence is highest in Pacific peoples (3.1 per 100,000, 11 cases in 2023) and Māori (2.1 per 100,000, 18 cases in 2023) compared with the total population. Household crowding is an important risk factor for meningococcal disease, independent of ethnicity. For 2023, the highest age-specific disease rates were among those aged under 1 year (12.2 per 100,000, seven cases) decreasing in ages 1–4 years (3.3 per 100,000, eight cases). One death occurred in 2023, giving a case fatality rate of 1.7 percent (ESR, 12 July 2024).
Strain types
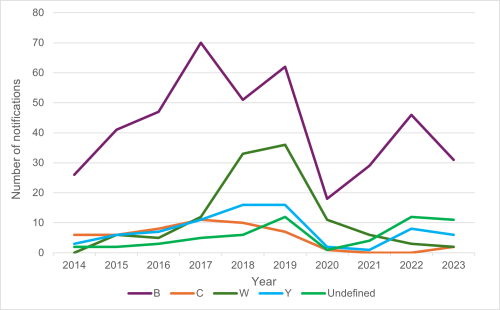
Strain type was determined for 41 of the 52 confirmed cases in 2023. In 2023, group B strains were the most prevalent (Figure 13.2). Out of 41 cases with the group identified, 31 (76 percent) were group B, six (15 percent) were group Y, two (5 percent) group W, and two (5 percent) group C.[13]
Within the group B cases, there were nine different PorA types identified. The most common were B:P1.7-12, 14 (11/31 cases, 35 percent) and the former epidemic strain B:P1.7-2,4 (8/31 cases, 26 percent).[13]
Cases of meningococcal disease caused by group C strains decreased since 2017 (Figure 13.2), while group W decreased from six cases in 2021 to three in 2022 and two in 2023. Cases in groups C, W and Y decreased in 2021, likely in part, due to the public health measures implemented to control the COVID-19 pandemic.
Figure 13.2: Meningococcal disease notifications by group, 2014–2023
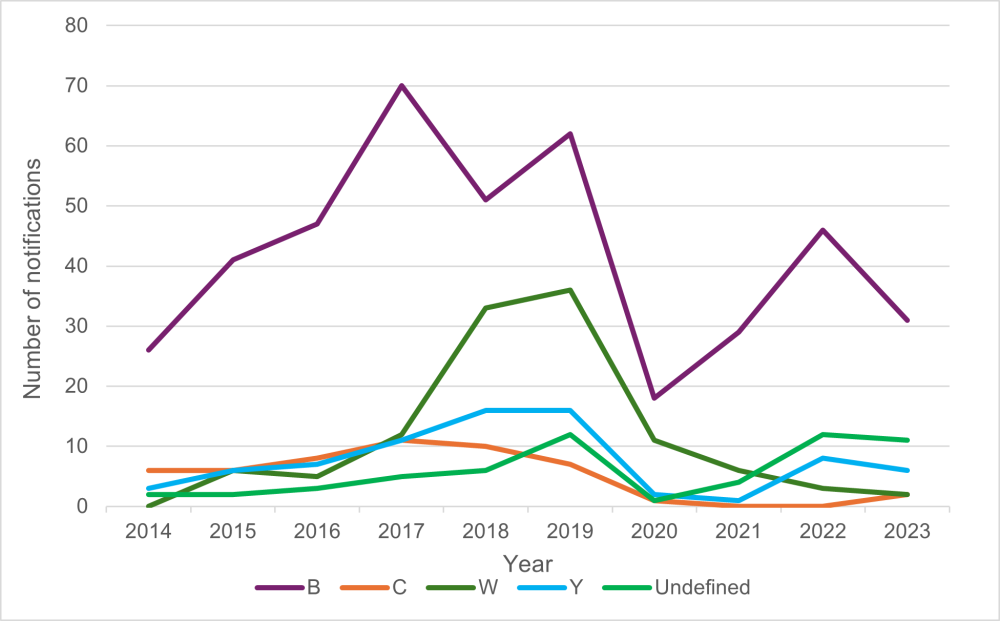
Source: ESR
13.4. Vaccines
13.4.1. Available vaccines
13.4.1. Available vaccines
Internationally, meningococcal vaccination programmes were revolutionised by the development of conjugate vaccines, which allow vaccination in younger children and induced herd immunity when used in population-wide programmes due to reduced nasopharyngeal carriage (see section 1.4.3).
The quadrivalent (ACWY) conjugate vaccines contain group A, C, W, Y polysaccharides conjugated to tetanus toxoid protein. Previously used, polysaccharide-only vaccines provided three to five years’ protection in adults, but they are generally regarded as inferior to conjugate vaccines and are no longer approved for use in New Zealand. Those travelling to Africa, the Middle East and other areas with wide serogroup prevalence, including group A, require MenACWY vaccine for broad protection. In 2018, a multicomponent meningococcal group B recombinant vaccine (MenB, formerly abbreviated 4CMenB) was registered in New Zealand to protect against group B disease.
With the current New Zealand epidemiology, neither MenACWY nor MenB give protection across all prevailing meningococcal groups and both types of vaccine are recommended. The meningococcal vaccines registered and available are summarised in Table 13.3 below.
Table 13.3: Meningococcal vaccines registered and available in New Zealand
|
Name (manufacturer) |
Vaccine type |
|---|---|
| Bexsero (GSK) |
Meningococcal group B four-component recombinant (MenB) |
| MenQuadfi (sanofi-aventis) |
Quadrivalent meningococcal conjugate (MenACWY-T): contains group A, C, W and Y polysaccharides conjugated to tetanus toxoid |
|
Nimenrix |
Quadrivalent meningococcal conjugate (MenACWY-T): contains group A, C, W and Y polysaccharides conjugated to tetanus toxoid |
Funded vaccines
Meningococcal B vaccine (Bexsero) is funded as part of the routine childhood Schedule for all infants. No meningococcal ACWY vaccines are currently on the routine Schedule. See section 13.5 for funded vaccines for special groups.
Three meningococcal vaccines are funded for certain groups (see section 13.5).
- Recombinant meningococcal B vaccine, MenB (Bexsero, GSK) contains four components from the group B meningococcus: three recombinant N. meningitidis group B surface proteins associated with bacterial adhesion and survival (Neisseria heparin binding antigen fusion protein, adhesin A protein, and factor H binding protein) plus detoxified outer membrane vesicles containing antigen as used in the MenNZB epidemic vaccine. Other components include aluminium hydroxide, sodium chloride, histidine and sucrose.
- Quadrivalent meningococcal conjugate vaccine MenACWY (MenQuadfi, sanofi-aventis). Each 0.5 mL dose contains 10 µg of each capsular polysaccharide, conjugated to 55 µg tetanus toxoid for each of the meningitidis group A, C, W and Y strains. Also contains sodium chloride, sodium acetate and water for injection. There is no preservative or adjuvant.
- Quadrivalent meningococcal conjugate vaccine, MenACWY (Nimenrix, Pfizer NZ). Each 0.5 mL contains 5 µg of each polysaccharide derived from the capsules of group A, C, W and Y N. meningitidis strains, conjugated to 44 µg of tetanus toxoid carrier protein. Other components and excipients include sodium chloride, trometamol and sucrose.
The tetanus toxoid-conjugated meningococcal MenACWY vaccine (MenQuadfi) replaced Menactra as the funded vaccine in mid-2023, for use in individuals aged 12 months and over. In July 2024, the monovalent MenC vaccine (NeisVac-C) was replaced by a tetanus toxoid-conjugated quadrivalent MenACWY vaccine, Nimenrix, funded for use in infants aged from 6 weeks to under 12 months with increased risk of meningococcal disease.
Other vaccines
A two-component meningococcal group B recombinant vaccine (MenB-fHbp; Trumenba, Pfizer) is licensed from 10 years of age, including in the US, Europe and Australia. A pentavalent meningococcal vaccine, MenACWY/MenB-fHbp (Penbraya, Pfizer), is also approve for use in those aged 10-25 years in the US (which contains the same components as Nimenrix and Trumenba). Neither vaccine is available in New Zealand.
Historic MeNZB vaccine
A strain-specific group B meningococcal vaccine (MeNZB, Chiron/Novartis) containing outer membrane vesicles derived from the epidemic strain B:4:P1.7b,4 (NZ 98/254) was developed for epidemic control in New Zealand and used between 2004 and 2008. The programme ceased in 2008 because of a decline in incidence of epidemic strain group B disease (see previous editions of the Handbook).
Since the immune response to MenNZB was short-lived, previous recipients who wish to be protected against meningococcal B disease will need to be fully immunised with MenB.
13.4.2. Efficacy and effectiveness
13.4.2. Efficacy and effectiveness
Meningococcal group B recombinant vaccine
Three years after initiation of the introduction of MenB to the national immunisation schedule in the UK, a 75 percent reduction in group B disease was reported in the vaccine-eligible age groups compared with a historical cohort.[14] With 88 percent coverage but a low number of cases (25), adjusted vaccine effectiveness for all group B strains was 59.1 percent (95% CI: -31.1–87.2) following two primary doses and one booster dose, with an estimated 277 cases prevented.[14] This research is ongoing.
Following MenB vaccination of adolescents aged 15-16 years in South Australian schools, there was an overall reduction of 71 percent (95% CI 15-90; p=0.02) in group B meningococcal disease cases aged 16–19 years: five cases in 2017–2018 (predicted 9.9 [95% predicted interval 3.9-17.5]) and one case in 2018–2019 (predicted 10.9 [4.4–19.1]).[15]
As was observed during college outbreaks in the US,[16, 17] in South Australia where MenB is administered in a school-based programme at age 15–18 years, MenB had no effect on disease-causing meningococcal carriage suggesting that vaccination of adolescents is unlikely to generate herd immunity.[18] A MenB vaccination campaign used during an isolated outbreak in a region of Québec, Canada outbreak demonstrated direct protection of 79 percent against outbreak strain group B disease and an overall impact of 86 percent in target groups with no herd effects.[19]
Cross-protection against meningococcal group W has been observed following vaccination of infants with MenB in the UK. Among age-cohorts that were fully and partially eligible to receive MenB, respectively, it was estimated that there were 69 percent (adjusted incidence rate ratio 0.31; 95% CI 0.2-0.67) and 52 percent (0.48; 0.28-0.81) fewer cases of group W disease than predicted in 2018/2019; this included direct and indirect protection over four years. An estimated 98 cases (95% CI 34-201) of group W disease were directly prevented in children aged under 5 years.[20] The researchers state that MenACWY conjugate vaccines would still be required for direct and indirect protection against those groups, since the degree of cross-protection is dependent on the expression of vaccine antigens on the meningococcal surface.[20]
There is limited data on its use in patients with chronic medical conditions and those immunocompromised by medication or disease, such as HIV infection or hereditary immune system defects. In a phase 3 clinical trial, children aged 2–17 years showed good but reduced immunogenicity in those with immunocompromise.[21] Immunogenicity in children with asplenia and splenic dysfunction was similar to healthy children but reduced in children with complement deficiencies.[22]
The safety and efficacy of MenB in adults above 50 years of age have not been established.
Quadrivalent meningococcal conjugate vaccines
Clinical trial data use immunogenicity and bactericidal antibody titres as a proxy for efficacy. Effectiveness of conjugate meningococcal vaccination against laboratory-confirmed disease is difficult to assess due to the low incidence of cases, even during localised epidemics, such that data is limited around the effectiveness of the MenACWY vaccines. With the emergence of group W and Y strains, more countries have implemented mass campaigns and routine immunisation programmes to control outbreaks with MenACWY vaccines and can assess impact through disease incidence and carriage studies. The current evidence indicates that MenACWY (MenQuadfi) has similar or superior immunogenicity to other MenACWY vaccines; real-world effectiveness data is limited to date as it was first licensed in 2020.
Several phase II and III clinical trials have assessed the immunogenicity of the MenACWY vaccine, MenQuadfi. These studies have included healthy vaccine-naive toddlers aged from 12 months; vaccine-naïve older children and adolescents; booster doses in toddlers, children and adolescents following prior MenACWY or MenC vaccination; given concurrently with routine vaccines (DTaP-IPV-HepB/Hib, MMR, VV, PCV13, Tdap and HPV); and in adults aged over 56 years.[23] In all studies, robust antibody responses were observed in all groups, immunogenicity was non-inferior to comparator vaccines and booster responses were observed.
Bactericidal antibody responses in adults (aged 18-55 years) were two to six times greater in those vaccinated with MenQuadfi, compared those vaccinated with diphtheria-toxoid conjugated MenACWY (Menactra), and a higher proportion of participants had a positive seroresponse.[24]
One clinical trial observed lower antibody responses to FHA, PRN and FIM2/3 pertussis antigens but not pertussis toxin, when MenACWY was given concurrently with Tdap and HPV in adolescents compared with without MenACWY.[25] The clinical relevance is unknown and has been observed with other MenACWY vaccines.[23] No interference was documented with other vaccines. There is no published data to date on effectiveness or use in immunocompromised individuals.
The other MenACWY-T vaccine (Nimenrix) is registered in New Zealand for individuals from aged 6 weeks. Clinical trials showed that the vaccine elicited bactericidal antibodies against all four groups from age 2 months with acceptable reactogenicity and safety profile.[26]
There is no published data on effectiveness in older adults for these vaccines.
13.4.3. Transport, storage and handling
13.4.3. Transport, storage and handling
Transport according to the National Standards for Vaccine Storage and Transportation for Immunisation Providers 2017 (2nd edition).
Store at +2°C to +8°C. MenACWY vaccines and MenB should be protected from light. Do not freeze.
Reconstitution
The MenACWY vaccine, Nimenrix, must be reconstituted with the supplied diluent and used as soon as possible. The other meningococcal vaccines are fully liquid formulations and do not require reconstitution.
13.4.4. Dosage and administration
13.4.4. Dosage and administration
Meningococcal group B recombinant vaccine (MenB)
Each MenB (Bexsero) dose is 0.5 ml, administered by intramuscular injection (see section 2.2.3).
- For infants aged 8 weeks to 11 months, two doses are given with a minimum of eight weeks between doses, with a booster given at least six months after the second dose, from age 12 months.
- For toddlers aged 12–23 months (at time of first dose) a primary course of two doses is given at least eight weeks apart. A booster dose is recommended from 12 to 23 months after dose two.
- For children aged from 2 years (at time of first dose) and adults, two doses are given at least eight weeks apart (see section 13.5.1 for reduced spacing). (Note: the safety and efficacy in individuals aged over 50 years have not been established, but no safety concerns are expected.)
Generally, the need for booster doses from the age 2 years or older at the time of their first dose has not been established. A booster dose can be considered for individuals at continued risk of exposure to meningococcal disease. Booster doses are funded five-yearly for some high-risk individuals (see Table 13.6).
MenB can be administered concurrently with other scheduled vaccines, in separate syringes and at separate sites (see section 2.2.7).
See section 13.5.1 for recommendations around antipyretic prophylaxis use with delivery in children aged under 2 years.
Quadrivalent meningococcal conjugate vaccines (MenACWY)
Each MenACWY dose is 0.5 mL, administered by intramuscular injection (see section 2.2.3).
MenACWY and MenC vaccines can be concurrently administered with other vaccines in separate syringes and at separate sites.
MenQuadfi
MenQuadfi is given as a single dose to individuals aged 12 months and over. See Table 13.6 for schedules and dosing for certain special groups.
A single booster dose may be given to adolescent and adults who have been primed with another MenACWY vaccine at least 4 years previously. There is currently no data available to indicate the need for or timing of a booster dose.
Nimenrix
Nimenrix is approved for use in New Zealand for individuals from age 6 weeks.
- For infants aged under 12 months, two doses are given eight weeks apart, plus a booster at least six months after second dose, from age 12 months. Primary doses are funded for certain infants aged 6 weeks to under 12 months (see Table 13.6).
- Healthy infants aged 6 months to under 12 months, who are not immunocompromised, can be given one dose (unfunded) instead of two primary doses, plus a booster from age 12 months, at least eight weeks later.
- For adults and children from age 12 months, one dose is given (unfunded).
- Booster doses may be indicated in some individuals.
13.5. Recommended immunisation schedule
13.5.1. Usual childhood schedule
13.5.1. Usual childhood schedule
A primary course of MenB is recommended and funded for all children at ages 3 months, 5 months and a booster given at age 12 months. In an alternative approved schedule, MenB can be given at ages 2 months (ie, approved from 8 weeks) and 4 months with a booster at age 12 months; this would be outside the standard scheduled immunisation visits. Do not delay the 6-week vaccination event for the other Schedule vaccines.
For children at high risk of meningococcal disease aged younger than 8 weeks, to align with the routine schedule visit, MenB can be given at ages 6 weeks and 3 months. This is off-label use and requires a prescription for both events. No prescription is required if given as per the alternative schedule at ages 2 and 4 months.
A catch-up programme of MenB is funded for children aged 13 months to 59 months from 1 March 2023 until 31 August 2025. All children eligible for meningococcal B catch-up should be vaccinated at the earliest opportunity. Give two doses 8 weeks apart. For children aged 12 to under 24 months at time of their first dose, a further dose can be given from 12 to 24 months after second dose. If MenB is started before the age 5 years, then the funded course can be completed.
As an antipyretic, prophylactic paracetamol is recommended to be given up to 30 minutes prior to or immediately after MenB vaccination and give up to two further doses four to six hours apart for children aged under 2 years. Ibuprofen may be given as an alternative to paracetamol six to eight hourly (see section 13.7.1).
Table 13.4: Recommended MenB immunisation schedule for children aged under 5 years
Table 13.4: Recommended MenB immunisation schedule for children aged under 5 years
|
Age |
Dose |
Comment |
|
National Immunisation Schedule |
||
|
3 monthsa |
Dose 1 |
Given together with routine vaccinations |
|
5 months |
Dose 2 |
|
|
12 months |
Dose 3 (booster) |
|
|
Approved alternative schedule |
||
|
8 weeksa |
Dose 1 |
Additional visit |
|
4 months |
Dose 2 |
Additional visit if dose one received at age 2 months |
|
12 months |
Dose 3 (booster) |
Given together with routine vaccinations |
|
Catch-up for those aged 12–59 months at time of first dose |
||
|
Age 12–23 months |
Give 2 doses at least 8 weeks apartb. Give a booster dose 12–24 months after dose two. |
|
|
Age 24–59 months |
Give 2 doses at least 8 weeksc apart, no booster. |
|
|
a. For children at high risk of meningococcal disease aged younger than 8 weeks, to align with the routine schedule visit, MenB can be given at ages 6 weeks and 3 months. This is off-label use and requires a prescription for both doses. No prescription is required if given as per the alternative schedule at ages 2 months (ie, 8 weeks) and 4 months. See Table 13.6 for high-risk infants. b. For children aged 8 weeks to under 24 months, spacing between dose one and two can be reduced to 6 weeks, if indicated with a prescription. c. For children aged 2 years and over, spacing between doses can be reduced to four weeks, if indicated. No prescription required. |
||
13.5.2. Individuals at increased risk
13.5.2. Individuals at increased risk
Meningococcal vaccines are funded in special circumstances, as described in the shaded section of Table 13.5; Table 13.6 shows the recommended dosing schedules.
See sections 4.2.5, 4.4 and 4.5 for more information about vaccination of special groups, including recommended immunisation schedules for high-risk individuals with certain medical conditions.
The meningococcal vaccines are recommended (but not funded) for other individuals at risk, as described in non-shaded rows in Table 13.5.
Before travel
There are areas of the world where the risk of meningococcal disease is increased. Nevertheless, the risk to travellers to the developing world has been estimated as being less than one in a million per month. Recurrent epidemics of meningococcal disease occur in the sub-Saharan ‘meningitis belt’, from Senegal in the west to Ethiopia in the east, usually during the dry season (December to June). Epidemics are occasionally identified in other parts of the world, including in Europe and the Americas. Generally, countries outside of Africa experience smaller outbreaks, but case-fatality rates can be high.
The preferred vaccines (MenACWY and/or MenB) for travel would be based on the epidemiology of the country. For website sources for information about meningococcal vaccines for travellers, see the WHO website. Quadrivalent meningococcal vaccine is a requirement for pilgrims to the Hajj.
Before moving into communal living situations
MenACWY and MenB are recommended and funded from age 13–25 years inclusively for individuals who will be living in communal accommodation within the next three months, or who are in their first year of living in communal accommodation (specifically, boarding school hostels, tertiary education halls of residence, military barracks, youth justice residences or prisons) as they are likely to be at higher risk of acquiring meningococcal infection. This includes children who turn 13 years of age while living in boarding school hostels.
Table 13.5: Meningococcal vaccine recommendations
Table 13.5: Meningococcal vaccine recommendations
| Note: Funded circumstances are in the shaded rows. See the Pharmaceutical Schedule for any changes to the funding decisions. |
|
Recommended and funded |
|---|
|
MenB is recommended and funded for:
MenB and MenACWY b are recommended and funded for:
MenB and MenACWY are recommended and funded for:
|
|
Recommended but not funded |
|
Priority groups MenACWY and MenB are recommended, but not funded, for:
Other groups MenACWY and MenB are recommended but not funded for all infantsh, young children, adolescents and young adults (see Table 13.7). |
|
a. For children at high risk of meningococcal disease aged younger than 8 weeks, to align with the routine schedule visit, MenB can be given at ages 6 weeks and 3 months. This is off-label use and requires a prescription for both doses. No prescription is required if given as per the alternative schedule at ages 8 weeks and 4 months. b. For these groups, Nimenrix is funded from age 6 weeks to <12 months; MenQuadfi at age 12 months and over. MenB is routinely funded for infants and catch-up is available for children aged 13 months to <5 years (until 31 August 2025). c. Pneumococcal, Hib, influenza and varicella vaccines are also recommended for individuals pre- or post-splenectomy or with functional asplenia. See section 4.3.3. d. See section 4.3.3 for more information. e. The period of immunosuppression due to steroid or other immunosuppressive therapy must be longer than 28 days. f. For close contacts and individuals who have had previous meningococcal disease, given as per the routine dosage schedule (see section 13.4.4). g. Regardless of time elapsed since disease. Vaccination can commence at any time from when the person is no longer acutely unwell. |
Table 13.6: Recommended meningococcal vaccine schedule for high-risk individuals (funded)
Table 13.6: Recommended meningococcal vaccine schedule for high-risk individuals (funded)
| Note: See the Pharmaceutical Schedule for any changes to funding decisions. |
|
Age at diagnosis |
Vaccine |
Recommended vaccine schedule |
|---|---|---|
|
Infants aged 6 weeks a,b to under 12 months |
MenACWY (Nimenrix) |
|
| MenB (Bexsero) a |
|
|
| Children aged 12 months to under 18 years | MenACWY (MenQuadfi) |
|
| MenB (Bexsero) |
|
|
| Adults aged 18 years and older | MenACWY (MenQuadfi) |
Give 2 doses 8 weeks apart, then 1 dose every 5 years. d |
| MenB g (Bexsero) | Give 2 doses 8 weeks apart e, then booster 5 yearly. d | |
| Individuals aged between 13 and 25 years, in certain communal living situations h | MenACWY (MenQuadfi) |
1 dose, no booster required h,i |
| MenB | Give 2 doses 8 weeks apart f | |
|
a. Nimenrix is approved for use from age 6 weeks. b. For children at high risk of meningococcal disease aged younger than 8 weeks, to align with the routine schedule visit, MenB can be given at ages 6 weeks and 3 months. This is off-label use and requires a prescription for both doses. No prescription is required if given as per the alternative approved schedule at ages 8 weeks and 4 months. c. For children given MenC prior to 1 July 2024, give two doses Nimenrix or MenQuadfi as age appropriate. d. MenACWY and MenB boosters are funded for individuals pre- and post-splenectomy and for patients with functional or anatomic asplenia, HIV, complement deficiency (acquired or inherited), or pre- or post-solid organ transplant. e. A second primary dose of MenACWY (MenQuadfi) from age 12 months is off-label and requires a prescription from an authorised prescriber under section 25 of the Medicines Act. f. From age 2 years, MenB can be given 4 weeks apart if there is a clinical need. g. MenB is licensed up to age 50 years, but no safety concerns are expected when given to adults aged over 50 years. h. Funded for individuals aged 13–25 years inclusively who either: are entering within three months or who are in their first year of living in boarding school hostels, tertiary education halls of residence, military barracks, youth justice residences or prisons. This includes those who turn 13 years of age while living in boarding school hostels. i. A single booster dose can be given to adolescents and young adults who were primed at least 4 years previously with any MenACWY vaccine. |
||
13.5.3. Recommendations for children and adolescents
13.5.3. Recommendations for children and adolescents
Non-high-risk children and adolescents may be offered meningococcal vaccines. These vaccines are only funded for certain groups (see Table 13.4). Table 13.7 suggests the most appropriate ages for this, reflecting the known ages of increased risk. The predominant meningococcal strains in New Zealand in childhood are B, W and Y. With the current New Zealand epidemiology, neither MenACWY nor MenB give protection across all prevailing meningococcal groups and both types of vaccine are recommended. For those who are likely to travel, the broadest protection is preferable because of the differing serotype patterns between countries.
Table 13.7: Recommended schedule for non-funded meningococcal vaccines in children and adolescents
| Note: Vaccine immunity is not long-lasting. The suggested ages of vaccination are not expected to protect individuals through all of childhood but focused on protection during the ages of highest risk. Due to being at lowest risk, healthy children aged 5–11 years are not specifically recommended meningococcal vaccination in this table. This does not apply to epidemic situations. |
|
Age at time of consultation |
Vaccine options |
Number of dosesa |
|---|---|---|
|
6 weeks to <12 months |
MenACWY (Nimenrix) |
ages 6 weeks–5 months give 2 doses plus a booster from age 12 months |
|
12 months to <5 years |
MenACWY |
1 dose MenACWY |
|
Early adolescence |
MenB (Bexsero) |
2 dosesd |
|
MenACWY |
1 dose plus a booster 5 years laterd |
|
|
Late adolescence |
MenB (Bexsero) |
2 doses |
|
MenACWY |
1 dose, no booster required |
|
|
a. Refer to section 13.4.4 for the intervals between doses. b. Infants aged from 6 months to ≤12 months who are not immunocompromised, can instead be given one dose plus booster from age 12 months at least two months later. c. For individuals aged 13–25 years not eligible for funded vaccine, particularly living in crowded private homes, other hostels or private student accommodation, or planning overseas travel. d. A booster dose of MenACWY and MenB can be given if it is at least 5 years since primary dose of these vaccines. The need for MenB vaccination is not established for this group but can be considered if at continued risk. |
||
13.5.4. Pregnancy and breastfeeding
13.5.4. Pregnancy and breastfeeding
13.5.5. (Re)vaccination
13.5.5. (Re)vaccination
Meningococcal conjugate vaccines, MenC, MenACWY and MenB are funded for vaccination or re-vaccination of eligible individuals, as follows. See also section 4.3.
Quadrivalent meningococcal conjugate vaccines, MenACWY
- Age under 12 months – up to three doses (Nimenrix), plus booster (MenQuadfi) after age 12 months and every five years (as appropriate)
- Age from 12 months – up to two doses (MenQuadfi) plus booster every five years (as appropriate)
Primary vaccination plus booster doses of MenACWY (as age appropriate) are funded for individuals:
- pre- or post-splenectomy
- with functional or anatomic asplenia
- pre- or post-solid organ transplantation
- with complement deficiency (acquired or inherited)
- who are HIV-positive.
A maximum of two (MenQuadfi) or three (Nimenrix, depending on age of first dose) doses are funded for individuals:
- post-haematopoietic stem cell transplantation
- prior to planned and following immunosuppression for longer than 28 days.
Recombinant meningococcal B vaccine, MenB
- Age under 12 months – up to three doses, plus booster after age 12 months and every five years (as appropriate)
- Age from 12 – 23 months – up to two doses plus booster given 12 to 23 months after second dose, and booster every five years (as appropriate)
- Age from 2 years – up to two doses plus a booster every five years (as appropriate)
Primary vaccination plus booster doses funded individuals:
- pre- or post-splenectomy
- with functional or anatomic asplenia
- pre- or post-solid organ transplantation
- with complement deficiency (acquired or inherited)
- who are HIV-positive.
Two primary doses are funded for individuals aged from 12 months:
- post-haematopoietic stem cell transplantation
- prior to planned and following immunosuppression for longer than 28 days.
13.6. Contraindications and precautions
See also section 2.1.3 for pre-vaccination screening guidelines and section 2.1.4 for general contraindications for all vaccines.
There are no specific contraindications for meningococcal vaccines, except for anaphylaxis to a previous dose or any component of the vaccine.
13.7. Potential responses and AEFIs
13.7.1. Meningococcal B recombinant vaccine
13.7.1. Meningococcal B recombinant vaccine
There is an increased risk of fever and medically attended fever-related events, such as sepsis screening and febrile seizures associated with MenB in some children age under 2 years.[4, 30, 31, 32, 33] These events peaked at six hours post-vaccination and generally subsided by day 3. Fever of at least 38°C was observed in 80 percent of infants who received MenB and routine vaccines concomitantly, and 71 percent who had MenB and routine vaccines separately, compared with 51 percent who receive routine vaccines only.[34, 35] Paracetamol was show to reduce the incidence of fever. Prophylactic paracetamol is recommended from 30 minutes prior to or immediately after vaccination, then two further doses can be given four to six hours apart for children aged under 2 years. Some infants will still develop a fever and/or injection-site pain even though they have received paracetamol doses.[36]
In clinical trials, most infants and young children had at least one solicited adverse reaction (84–97 percent)[35], including injection-site tenderness and irritability. Adolescents and adults may experience localised pain, nausea, myalgia, malaise, mild fever and headache.
13.7.2. Quadrivalent meningococcal conjugate vaccine
13.7.2. Quadrivalent meningococcal conjugate vaccine
Potential adverse reactions after meningococcal conjugate vaccines include localised injection-site pain, irritability, headache, malaise and fatigue.[26, 37] The safety profile of all the MenACWY vaccines are very similar.[26, 27, 28] In infants, unusual crying, irritability, drowsiness and vomiting have been reported in clinical trials.[24, 39] Fever is reported by 2–5 percent of adolescents and less than 10 percent of infants who receive MenACWY. Concurrent administration with routine vaccines did not increase solicited systemic responses in young children (when given with PCV13) but was slightly increased in adolescents (with Tdap and HPV) during clinical trials of MenQuadfi.[24, 38, 39]
Guillain–Barré syndrome
There is no evidence of an association between meningococcal conjugate vaccines and GBS. An early report in the US of a suspected temporal association between MenACWY (Menactra) and GBS was followed by a large retrospective cohort study in the US that found no evidence of an increased risk of GBS following administration of MenACWY.[28, 40] If indicated, meningococcal conjugate vaccines may be administered to individuals with a history of GBS.[41]
13.8. Public health measures
Invasive meningococcal disease must be notified on suspicion to the local medical officer of health.
For details of public health control measures see the Communicable Disease Control Manual.
The overall rate of secondary cases in untreated adults is around 1 per 300. Adults and children in close contact with primary cases of invasive meningococcal infection are recommended to receive antibiotic prophylaxis, preferably within 24 hours of the initial diagnosis, but prophylaxis is recommended up to 14 days after diagnosis of illness.
13.8.1. Use of meningococcal vaccines for close contacts
13.8.1. Use of meningococcal vaccines for close contacts
Close contacts of cases of any group (including group A, B, C, W or Y) meningococcal disease may be offered both MenB and MenACWY meningococcal vaccines (see section 13.5).
See below for the use of the vaccines for the control of outbreaks, as initiated by the local public health service.
13.8.2. Outbreak control
13.8.2. Outbreak control
When there is an outbreak of meningococcal disease of a specific vaccine group, an immunisation programme may be recommended and funded for a defined population. The local medical officer of health will determine the necessary action in discussion with Health New Zealand.
For more details on control measures, refer to the ‘Neisseria meningitidis invasive disease’ chapter of the Communicable Disease Control Manual .
13.9. Variations from the vaccine data sheets
The MenQuadfi data sheet states to give a single dose for individuals from age 12 months. Health New Zealand | Te Whatu Ora recommends that two doses are given to individuals at high risk of meningococcal disease (see Table 13.6 and section 4.2.5), with booster doses every five years. If the child is still under the age of 7 years, give a booster after three years then five-yearly.[37] The second primary dose of MenQuadfi requires a prescription from an authorised prescriber.
The MenB data sheet states that the vaccine is indicated from age 8 weeks or older. However, Health NZ recommends that MenB can be given from age 6 weeks to infants at high risk of meningococcal disease (see Table 13.6). This is an off-label use and requires a prescription from an authorised prescriber. The datasheet states that infants who received their primary course from ages 6 to 11 months can receive a booster in the second year of life at least 2 months later. The Ministry recommends that all infants aged 11 months or less receive a booster dose at least 6 months after the primary doses, from the age of 12 months.
The data sheet recommends two doses of MenB to be given eight weeks apart between ages 12 and 23 months and not less than one month apart from the age of 2 years. Health NZ recommends two doses of MenB be given at least eight weeks apart for those aged 12 months or older at the time of the first dose, this may be reduced to at least 4 weeks if required.
References
References
References
- Campbell H, Parikh SR, Borrow R, et al. Presentation with gastrointestinal symptoms and high case fatality associated with group W meningococcal disease (MenW) in teenagers, England, July 2015 to January 2016. Euro Surveill, 2016. 21(12).
- Ladhani SN, Beebeejaun K, Lucidarme J, et al. Increase in endemic Neisseria meningitidis capsular group W sequence type 11 complex associated with severe invasive disease in England and Wales. Clin Infect Dis, 2015. 60(4): p. 578-85.
- Cohn A ,MacNeil J. The Changing Epidemiology of Meningococcal Disease. Infect Dis Clin North Am, 2015. 29(4): p. 667-77.
- Harrison LH, Granoff DM ,Pollard AJ. 2018. Meningococcal Capsular Group A, C, W and Y conjugate vaccines, in Plotkin's Vaccines (7th edition), Plotkin SA, Orenstein W, Offit P, and Edwards K (eds). Elsevier: Philadelphia, US.
- Rubilar PS, Barra GN, Gabastou JM, et al. Increase of Neisseria meningitidis W:cc11 invasive disease in Chile has no correlation with carriage in adolescents. PLoS One, 2018. 13(3): p. e0193572.
- Wang B, Santoreneos R, Giles L, et al. Case fatality rates of invasive meningococcal disease by serogroup and age: A systematic review and meta-analysis. Vaccine, 2019. 37(21): p. 2768-2782.
- Meningococcal Disease Surveillance Group. Analysis of endemic meningococcal disease by serogroup and evaluation of chemoprophylaxis. J Infect Dis, 1976. 134(2): p. 201-4.
- Neal KR, Nguyen-Van-Tam JS, Jeffrey N, et al. Changing carriage rate of Neisseria meningitidis among university students during the first week of term: cross sectional study. BMJ, 2000. 320(7238): p. 846-9.
- Bruce MG, Rosenstein NE, Capparella JM, et al. Risk factors for meningococcal disease in college students. JAMA, 2001. 286(6): p. 688-93.
- Nelson SJ, Charlett A, Orr HJ, et al. Risk factors for meningococcal disease in university halls of residence. Epidemiol Infect, 2001. 126(2): p. 211-7.
- Christensen H, May M, Bowen L, et al. Meningococcal carriage by age: a systematic review and meta-analysis. The Lancet Infectious Diseases, 2010. 10(12): p. 853-61.
- Peterson ME, Li Y, Shanks H, et al. Serogroup-specific meningococcal carriage by age group: a systematic review and meta-analysis. BMJ Open, 2019. 9(4): p. e024343.
- Institute of Environmental Science and Research (ESR). 2024 Invasive meninogoccal disease report, January - December 2023. ESR: Porirua, New Zealand. URL: www.esr.cri.nz/meningococcal-reports
- Ladhani SN, Andrews N, Parikh SR, et al. Vaccination of Infants with Meningococcal Group B Vaccine (4CMenB) in England. N Engl J Med, 2020. 382(4): p. 309-317.
- McMillan M, Wang B, Koehler AP, et al. Impact of Meningococcal B Vaccine on Invasive Meningococcal Disease in Adolescents. Clinical Infectious Diseases, 2021.
- McNamara LA, Thomas JD, MacNeil J, et al. Meningococcal Carriage Following a Vaccination Campaign With MenB-4C and MenB-FHbp in Response to a University Serogroup B Meningococcal Disease Outbreak-Oregon, 2015-2016. J Infect Dis, 2017. 216(9): p. 1130-1140.
- Soeters HM, Whaley M, Alexander-Scott N, et al. Meningococcal carriage evaluation in response to a serogroup B meningococcal disease outbreak and mass vaccination campaign at a college – Rhode Island, 2015 – 2016. Clin Infect Dis, 2017. 64(8): p. 1115-1122.
- Marshall HS, Lally N, Flood L,Phillips P. First statewide meningococcal B vaccine program in infants, children and adolescents: evidence for implementation in South Australia. Med J Aust, 2020.
- Deceuninck G, Lefebvre B, Tsang R, et al. Impact of a mass vaccination campaign against Serogroup B meningococcal disease in the Saguenay-Lac-Saint-Jean region of Quebec four years after its launch. Vaccine, 2019. 37(31): p. 4243-4245.
- Ladhani SN, Campbell H, Andrews N, et al. First real world evidence of meningococcal group B vaccine, 4CMenB, protection against meningococcal group W disease; prospective enhanced national surveillance, England. Clin Infect Dis, 2020.
- GlaxoSmithKline NZ Ltd, New Zealand Data sheet: Bexsero multicomponent meningococcal group B vaccine (recombinant, adsorbed). 2020, Medsafe.
- Martinón-Torres F, Bernatowska E, Shcherbina A, et al. Meningococcal B vaccine immunogenicity in children with defects in complement and splenic function. Pediatrics, 2018. 142(3).
- Martinón-Torres F, Bertrand-Gerentes I ,Oster P. A novel vaccine to prevent meningococcal disease beyond the first year of life: an early review of MenACYW-TT. Expert Review of Vaccines, 2021. 20(9): p. 1123-1146.
- sanofi-aventis New Zealand. New Zealand datasheet: MenQuadfi. Medsafe; [updated 22 September 2022]; URL: https://www.medsafe.govt.nz/Profs/Datasheet/m/Menquadfiinj.pdf. (accessed 1 December 2022)
- Chang LJ, Hedrick J, Christensen S, et al. A Phase II, randomized, immunogenicity and safety study of a quadrivalent meningococcal conjugate vaccine, MenACYW-TT, in healthy adolescents in the United States. Vaccine, 2020. 38(19): p. 3560-3569.
- Hedari CP, Khinkarly RW ,Dbaibo GS. Meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate vaccine: a new conjugate vaccine against invasive meningococcal disease. Infect Drug Resist, 2014. 7(3 April): p. 85-99.
- Mbaeyi SA, Bozio CH, Duffy J, et al. Meningococcal Vaccination: Recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep, 2020. 69(9): p. 1-41.
- Centers for Disease Control and Prevention. 2013. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report: Recommendations and Reports. 62(2): p. 1–28. https://www.cdc.gov/mmwr/pdf/rr/rr6202.pdf (accessed 27 February 2020)
- Drugs and Lactation Database (LactMed). 2006-. Meningococcal Vaccines. in Drugs and Lactation Database (LactMed). Bethesda MD. URL: https://www-ncbi-nlm-nih-gov.ezproxy.auckland.ac.nz/books/NBK501014/. (accessed 1 December 2022)
- Vesikari T, Esposito S, Prymula R, et al. Immunogenicity and safety of an investigational multicomponent, recombinant, meningococcal serogroup B vaccine (4CMenB) administered concomitantly with routine infant and child vaccinations: results of two randomised trials. The Lancet, 2013. 381(9869): p. 825-35.
- Vesikari T, Prymula R, Merrall E, et al. Meningococcal serogroup B vaccine (4CMenB): Booster dose in previously vaccinated infants and primary vaccination in toddlers and two-year-old children. Vaccine, 2015. 33(32): p. 3850-8.
- Harcourt S, Morbey RA, Bates C, et al. Estimating primary care attendance rates for fever in infants after meningococcal B vaccination in England using national syndromic surveillance data. Vaccine, 2018. 36(4): p. 565-571.
- Zafack JG, Bureau A, Skowronski DM,De Serres G. Adverse events following immunisation with four-component meningococcal serogroup B vaccine (4CMenB): interaction with co-administration of routine infant vaccines and risk of recurrence in European randomised controlled trials. BMJ Open, 2019. 9(5): p. e026953.
- Gossger N, Snape MD, Yu L-M, et al. Immunogenicity and tolerability of recombinant serogroup B meningococcal vaccine administered with or without routine infant vaccinations according to different immunization schedules. JAMA, 2012. 307(6).
- Martinón-Torres F, Safadi MAP, Martinez AC, et al. Reduced schedules of 4CMenB vaccine in infants and catch-up series in children: Immunogenicity and safety results from a randomised open-label phase 3b trial. Vaccine, 2017. 35(28): p. 3548-3557.
- De Serres G, Billard MN, Gariépy MC, et al. Short-term safety of 4CMenB vaccine during a mass meningococcal B vaccination campaign in Quebec, Canada. Vaccine, 2018. 36(52): p. 8039-8046.
- American Academy of Pediatrics. 2018. Meningococcal disease. in Red Book: 2018 Report of the Committee on Infectious Diseases, Kimberlin D, Brady M, Jackson M, and Long S (eds). Elk Grove Village, IL. p. 551-561. URL: https://redbook.solutions.aap.org/redbook.aspx. (accessed 15 April 2020)
- Pina LM, Bassily E, Machmer A, et al. Safety and immunogenicity of a quadrivalent meningococcal polysaccharide diphtheria toxoid conjugate vaccine in infants and toddlers: three multicenter phase III studies. Pediatric Infectious Disease Journal, 2012. 31(11): p. 1173-83.
- Marshall GS, Pelton SI, Robertson CA,Oster P. Immunogenicity and safety of MenACWY-TT, a quadrivalent meningococcal tetanus toxoid conjugate vaccine recently licensed in the United States for individuals ≥2 years of age. Hum Vaccin Immunother, 2022: p. 2099142.
- Velentgas P, Amato AA, Bohn RL, et al. Risk of Guillain-Barré syndrome after meningococcal conjugate vaccination. Pharmacoepidemiol Drug Saf, 2012. 21(12): p. 1350-8.
- Australian Technical Advisory Group on Immunisation. 2018. Australian Immunisation Handbook (ed.), Canberra: Australian Government Department of Health. URL: https://immunisationhandbook.health.gov.au/ (accessed October 2019)