On this page
Key information
|
Mode of transmission |
By aerosolised droplets. |
|
|---|---|---|
|
Incubation period |
7–10 days (range 5–21 days). |
|
|
Period of communicability |
For control purposes, in untreated cases the communicable stage lasts from the catarrhal stage, approximately 1 week before, to 3 weeks after the onset of paroxysmal cough. When treated communicability lasts approximately 2–5 days from the first dose. |
|
|
Incidence and burden of disease |
Widespread outbreaks occur every 3–5 years. Infants aged under 12 months are at highest risk from pertussis, particularly those who have received fewer than two doses of vaccine and if the mother missed receiving vaccine in pregnancy. |
|
|
Funded vaccines |
DTaP-IPV-HepB/Hib (Infanrix-hexa). DTaP-IPV (Infanrix-IPV). Tdap (Boostrix). |
|
|
Dose, presentation, route |
0.5 mL per dose. DTaP-IPV-HepB/Hib: pre-filled syringe and glass vial. The vaccine must be reconstituted prior to injection. DTaP-IPV, Tdap: pre-filled syringe. Intramuscular injection. |
|
|
Funded vaccine indications and schedule |
During each pregnancy (recommended from 16 weeks’ gestation) |
Tdap |
|
6 weeks, 3 months and 5 months |
DTaP-IPV-HepB/Hib |
|
|
4 years |
DTaP-IPV |
|
|
11 years |
Tdap |
|
|
45 years (catch-up, if individual has not received 4 previous tetanus doses) |
Tdap |
|
|
65 years |
Tdap |
|
|
Parents or primary caregivers of infants admitted to neonatal intensive or special care baby units for more than 3 days and whose mothers had not received Tdap at least 14 days prior to birth |
Tdap |
|
|
For vaccination of previously unimmunised or partially immunised patients |
DTaP‑IPV‑HepB/Hib, DTaP-IPV or Tdap |
|
|
For (re)vaccination of eligible patients |
||
|
Recommendations, not funded |
|
|
|
Vaccine effectiveness |
Vaccination in pregnancy is over 90 percent effective in preventing pertussis in infants up to age 3 months. A 3-dose primary course in infants has 84 percent efficacy against hospitalisation for pertussis. |
|
|
Contraindications |
Contraindicated where anaphylaxis to vaccine or vaccine components is proven. |
|
|
Potential responses to vaccine |
Extensive limb swelling occurs more commonly after increasing number of doses of DTaP. Affecting less than in 2 percent of children, this is typically painless and resolves spontaneously. |
|
|
Public health measures |
Notify the local medical officer of health immediately on suspicion of wild-type pertussis. (see section 16.8) |
|
|
Post-exposure prophylaxis |
For management of contacts, see section 16.8. |
|
16.1. Bacteriology
Pertussis (whooping cough) is a bacterial respiratory infection caused by Bordetella pertussis, an exotoxin-producing gram-negative bacillus. The bacillus is fastidious (requires special techniques to grow in culture) and will often have decreased in numbers by the time the typical cough develops, making laboratory confirmation by culture difficult. The availability of sensitive and specific PCR and serological assays has improved laboratory confirmation of suspected B. pertussis infection (see section 16.8).
16.2. Clinical features
Pertussis is highly transmissible. It is one of the most infectious vaccine-preventable diseases in humans. The rate of transmission is several-fold greater than most respiratory pathogens, including influenza, such that in a non-immune population, approximately 5–17 secondary pertussis cases are expected from one case (see section 1.2.1 (external link)).[1] Transmission occurs by aerosolised droplets, and the incubation period is 7–10 days (range 5–21 days).
Pertussis is primarily a toxin-mediated disease. The exotoxin produced by B. pertussis paralyses the cilia, and causes inflammation of the respiratory tract, which interferes with the clearing of pulmonary secretions and results in the symptoms associated with pertussis.
There are three stages of typical pertussis infection:
- Catarrhal stage – rhinorrhoea and irritating mild cough (typically lasting 7–10 days).
- Paroxysmal stage – paroxysms (bursts) of coughing; in children, these may end in vomiting, cyanosis or apnoea and inspiratory gasp or whoop (1–6 weeks). Usually afebrile.
- Convalescent stage – less persistent cough, gradual recovery (up to 10 weeks).
Pertussis is highly communicable in the catarrhal stage and during the first two weeks of the paroxysmal stage. For control purposes, the communicable period lasts from the catarrhal stage to 21 days after the onset of cough, if untreated with an effective antibiotic. See the Communicable Disease Control Manual at https://www.tewhatuora.govt.nz/for-health-professionals/clinical-guidance/communicable-disease-control-manual/pertussis. (external link)
Clinical presentation varies with age, immunisation status and previous infection. Pertussis must be considered in infants presenting with apnoea, since apnoea and/or cyanosis may precede paroxysmal cough.[2] In school-aged children, inspiratory whoop, post-tussive vomiting and the absence of wheezing and fever distinguish pertussis from other causes of coughing illnesses.[3, 4] Almost all pertussis infections in adolescents and adults occur in the context of previous infection and/or immunisation and can present with illness ranging from a mild cough to classic pertussis. Persistent cough for more than 14 days is the cardinal feature in adults.[4, 5, 6] Coughing is often paroxysmal and worsens at night, with the patient waking with a choking sensation, but post-tussive vomiting and whoop are infrequent.
Studies performed in several countries during both epidemic and non-epidemic periods have shown that between 12 and 37 percent of school-aged children, adolescents and adults with persistent cough (lasting 14 days or more) have evidence of recent B. pertussis infection.[3, 5, 7, 8, 9] A primary care-based study in New Zealand performed during the early phase of the 2011–2013 epidemic showed recent B. pertussis infection in 17 percent of children aged 5–16 years and 7 percent of adults aged 17–49 years presenting to primary care with a persistent cough of two or more weeks’ duration.[10] These age groups are an important reservoir of B. pertussis and often the source of infection for infants.
The disease is most often severe in infants in the first few months of life. One in six infants with pertussis sufficiently severe to require intensive care admission will either die or be left with brain or lung damage.[11 (external link)] The most common complications of pertussis are secondary infections, such as otitis media and pneumonia, and the physical sequelae of paroxysmal coughing, (eg, petechiae and other haemorrhages within subconjunctiva, nasopharynx and central nervous system; pneumothorax; hernia; and urinary incontinence). At the peak of the paroxysmal phase, vomiting can lead to weight loss especially in infants and young children.
16.3. Epidemiology
The epidemiology of B. pertussis infection and pertussis disease differ. Infection occurs across the age spectrum, and repeated infection without disease is common.[12] The endemic circulation of B. pertussis in older children and adults provides a reservoir for spread of the infection and the development of severe disease in incompletely vaccinated infants. The high prevalence of subclinical infections in household contacts of pertussis cases indicates a significant role in disease transmission to young infants.[13, 14] As observed in Australia, seasonal peaks in incidence in children aged less than 5 years occurred 1–2 months later than for the general population, supporting the theory that older household members are sources of infection to younger children.[15]
16.3.1. Global burden of disease
16.3.1. Global burden of disease
Pertussis mortality and morbidity rates continue to be highest in the first year of life.[13] In the US during the 1940s pertussis resulted in more infant deaths than measles, diphtheria, poliomyelitis and scarlet fever combined.[16] Beyond age 3 years mortality rates have always been relatively low. In immunised populations virtually all deaths occur in the first two months of life, and deaths in toddlers and preschool-aged children have largely disappeared. Among infants, younger age, lack of immunisation, low socioeconomic status, premature gestation, low birthweight and female gender are associated with an increased risk of fatal pertussis.[17]
Pertussis mortality and morbidity is under-reported.[18, 19] It is estimated that there are three times more deaths due to pertussis than are reported in high-income countries.[18, 20, 21] The burden of pertussis in older adults is underestimated, particularly for those with chronic respiratory conditions, and increases with age.[22, 23] Infants continue to die from pertussis despite advances in intensive care.[11, 24, 25, 26]
Following the introduction of mass immunisation, countries with consistently high immunisation coverage rates have achieved consistently low pertussis incidence rates.[27] The most pronounced decrease in incidence was seen in those aged under 10 years. Although primarily associated with low immunisation coverage, in some instances higher pertussis incidence rates are due to lower or waning vaccine efficacy or less-than-optimal immunisation schedules.[28, 29, 30] The burden of severe disease, particularly since the introduction of acellular vaccines, is highest in infants and unvaccinated young children.[31] However, less severe pertussis cases are also seen in vaccinated children who are further away from the last DTaP and, in some countries, adolescents.[32, 33, 34] Infants too young to have received more than one dose of pertussis vaccine (age 3 months or less) have the highest rate of notification, hospitalisation and death.[35, 36]
Epidemic peaks of pertussis occur every 2–5 years without the consistent seasonal pattern that is typical of most respiratory infections, although evidence from Australia suggests increased incidence (by 15 percent compared with annual average) during spring to summer months.[15] Epidemics are frequently sustained over 18 months or more, during which there are dramatic increases in hospital admission rates. Lack of change in the pertussis epidemic cycle with mass immunisation suggests minimal impact on the circulation of B. pertussis in the population, unlike other epidemic vaccine-preventable diseases, such as measles.[12, 19, 37 (external link)]
16.3.2. New Zealand epidemiology
16.3.2. New Zealand epidemiology
Pertussis mortality in New Zealand
On average, zero to one deaths are associated with pertussis each year in New Zealand. During the 2011–2013 pertussis epidemic there were three deaths in children: two in infants aged under 6 weeks and one in an unimmunised pre-schooler.[38] No deaths from pertussis (as recorded in EpiSurv) occurred during the latest epidemic from October 2017 to May 2019.[38, 39]
Pertussis morbidity in New Zealand
Pertussis morbidity in New Zealand has usually been described using hospital discharge data. National passive surveillance data has been available since 1996, when pertussis became a notifiable disease.
Outbreaks continue to occur throughout New Zealand. For further details refer to the PHF Science (formerly ESR) surveillance reports for notifiable diseases and pertussis dashboard (external link).
Pertussis morbidity in New Zealand as described by notification data
Five epidemics have occurred since pertussis became a notifiable disease, with peaks in notified cases in 2000, 2004, 2012, 2018 and 2024, and total notified cases per epidemic year of around 3,000 to almost 6,000 (see Figure 16.1 to show recent peaks). During the period of COVID-19 pandemic control measures, between 2020 and 2023, very few cases of pertussis were notified. However, in early 2023, three infants younger than 8 weeks old died of pertussis without evidence of wider community cases.
Figure 16.1: Pertussis notifications by month January 2010 to February 2025
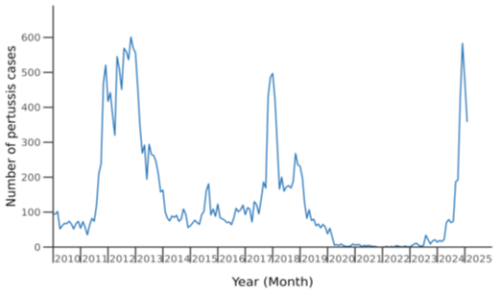
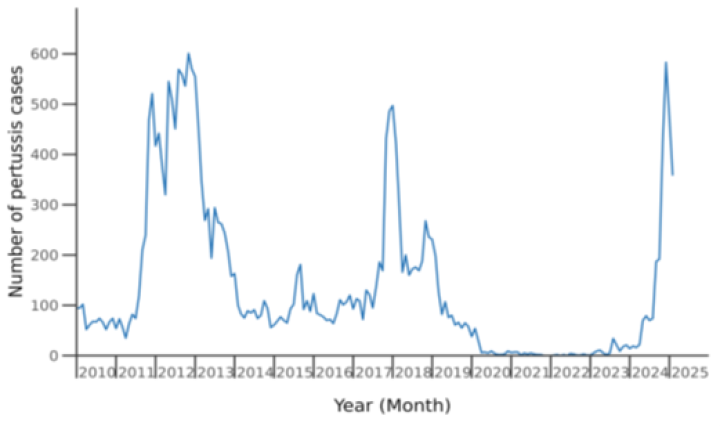
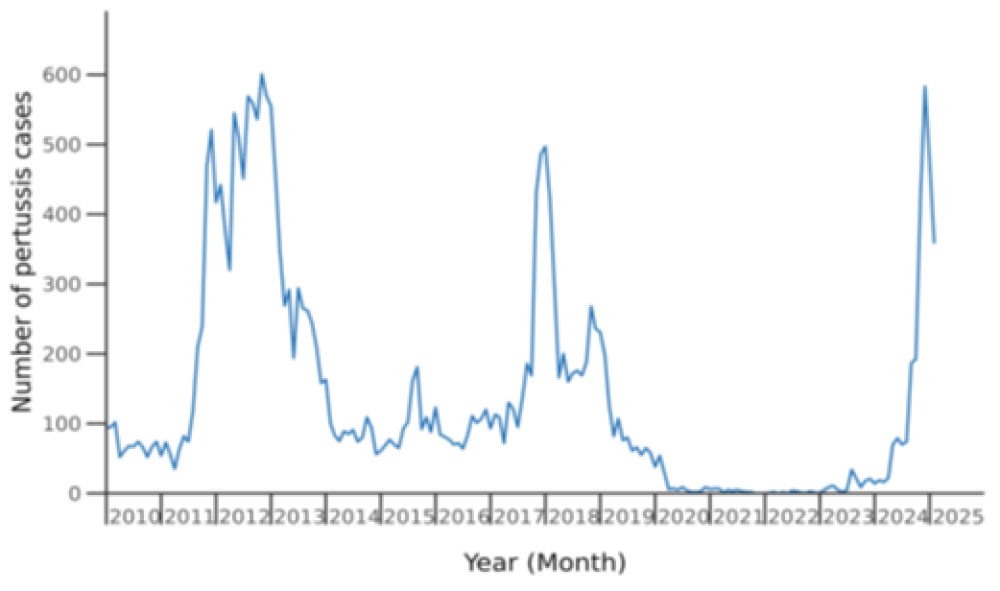
Source: ESR pertussis report, 8 Feb – 7 March 2025
From mid-2024, a sustained increase in case numbers began in October and on 22 November 2024, a pertussis outbreak was declared with a total 1,749 cases in 2024 (overall notification rate of 33 per 100,000 population). One young infant aged less than 8 weeks died. People of Māori ethnicity also had the highest rates of pertussis hospitalisations overall (45 cases per 100,000).
From 2010 to 2019 there were 1,544 notified cases in infants with 769 (53 percent) recorded as hospitalised (Figure 16.2). From 19 October 2024 to 7 March 2025, 82 out of 147 (55.8%) cases in infants aged <1 year were hospitalised in comparison to 7.7% of cases aged 1-4 years. (See PHF Science (formerly ESR) pertussis reports). The highest proportion of hospitalised cases continues to be in infants. In 2025, infants aged 0-5 months had the highest rates of notifications (236 per 100,000) and of hospitalisations (192 per 100,000).(PHF Science (formerly ESR) digital library)
Figure 16.2: Age distribution of notified and hospitalised pertussis cases, 2019
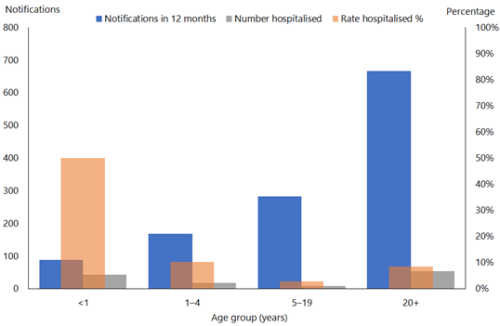
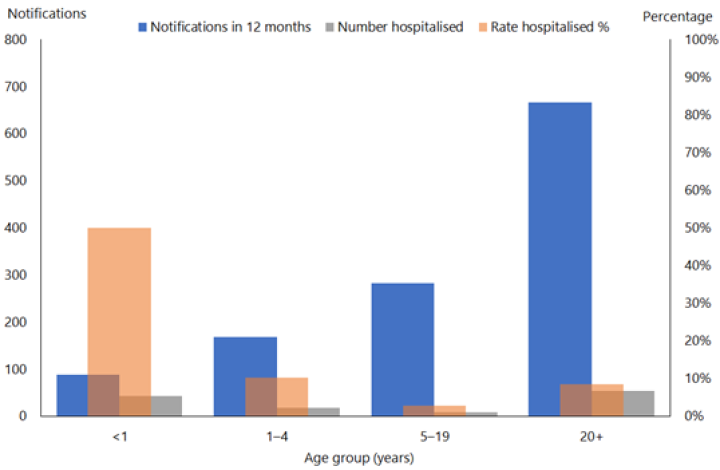
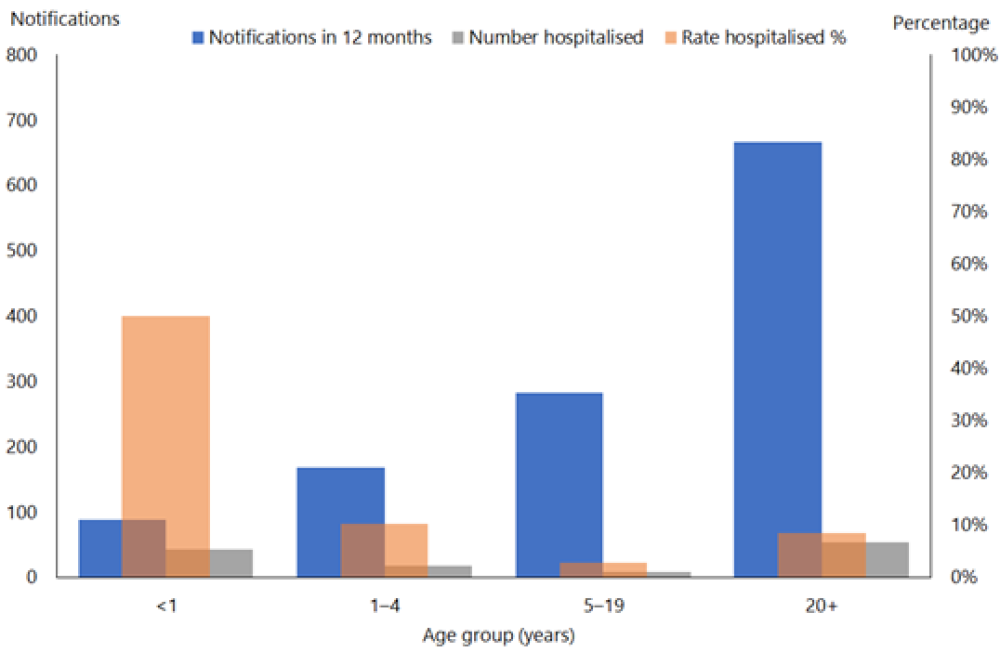
Source: ESR
Pertussis morbidity in New Zealand, as described by hospital discharge data
Infants aged under 1 year with pertussis are more likely (nearly 80 percent) to be admitted to hospital than older children and account for almost all the pertussis cases admitted to the paediatric intensive care unit.[40]
Hospitalisation rates for pertussis, as measured by ICD discharge diagnostic codes, provide a measure of severe pertussis disease. The discharge rate in the 2000s was lower than it was in the 1990s (2000s versus 1990s, relative risk 0.8 [95% CI: 0.7–0.8]). Despite this decrease, the infant hospitalisation rate for pertussis in New Zealand in the 2000s (at nearly 200 per 100,000) remained three times higher than contemporary rates in Australia (2001 infant rate: 56 per 100,000) and the US (2003 infant rate: 65 per 100,000).[41, 42, 43]
Pertussis hospital admission rates vary with ethnicity and household deprivation. From 2000–2014 the infant (under 1 year old) hospitalisation rates for pertussis fluctuated but were consistently higher for Pacific and Māori than European/Other prioritised ethnicities. Between 2010 and 2014, the hospitalisation rate was over 2.5 times higher for Pacific (4.4 per 1,000) and over 2 times higher for Māori (3.6 per 1,000) than it was for European/Other ethnicities (1.7 per 1,000).[44]
From 2010 to 2014 an infant living in a household in the most deprived quintile was at a five-fold increased risk of being hospitalised with pertussis compared with an infant in the least deprived quintile (4.0 versus 0.8 per 1,000).[44]
16.4. Vaccines
Whole-cell pertussis vaccine for routine use was introduced in 1960 and was replaced with acellular pertussis vaccine in 2000. The current schedule of three acellular pertussis-containing vaccines in the first year of life plus booster doses at ages 4 and 11 years has been in effect since 2006. See Appendix 1 for more information about the history of pertussis-containing vaccines in New Zealand.
16.4.1. Available vaccines
16.4.1. Available vaccines
Funded pertussis vaccines
The acellular pertussis-containing vaccines funded as part of the Schedule are:
- DTaP-IPV-HepB/Hib (Infanrix-hexa, GSK): diphtheria, tetanus, acellular pertussis, inactivated polio, hepatitis B and Haemophilus influenzae type b vaccine
- DTaP-IPV (Infanrix-IPV, GSK): diphtheria, tetanus, acellular pertussis and inactivated polio vaccine
- Tdap (Boostrix, GSK): a smaller adult dose of diphtheria and pertussis vaccine, together with tetanus vaccine.
See section 6.4.1 for more details.
Other vaccines
Other acellular pertussis-containing vaccines registered (approved for use) and available (marketed) in New Zealand include:
- Tdap: Adacel (Sanofi)
- Tdap-IPV: Adacel Polio (Sanofi).
16.4.2. Efficacy and effectiveness
16.4.2. Efficacy and effectiveness
Immunogenicity
A review of published data on DTaP-IPV-HepB/Hib found it to be highly immunogenic in infants aged under 2 years for primary and booster vaccination.[45] In clinical studies there was a strong immune response against the vaccine antigens, which persisted for up to approximately six years after vaccination. A review of published clinical trial and post-marketing surveillance data supported the immunogenicity of DTaP‑IPV-HepB/Hib across a range of schedules and when administered concurrently with other vaccines.[46]
Efficacy and effectiveness
Vaccination in pregnancy
Maternal vaccination, given more than seven days before delivery, was estimated to be 91 percent (95% CI: 88–94) effective against laboratory-confirmed pertussis in infants younger than 3 months of age.[47] Protection of infants is achieved both by passive antibody transfer and reduced exposure to maternal disease.[48] Tdap given in pregnancy was shown to be 85 percent more effective than post-partum vaccination in preventing pertussis in infants younger than 8 weeks of age.[49]
Timing is important because protection is not as good if the mother is vaccinated less than two weeks prior to birth.[50] Vaccinating from 16 weeks’ gestation allows time for passive transfer and accumulation of antibody in the fetus, such that by 40 weeks’ gestation, infant antibody levels at birth are higher than those in the mother.[51] Giving maternal vaccination during the second trimester rather than later provides more preterm infants with pertussis protection.[52, 53]
See section 16.5.2 for information about maternal pertussis vaccine safety.
Direct protection
The acellular pertussis vaccines approved for use in New Zealand have been shown to provide around 81–85 percent efficacy (95% CI: 51–100) against confirmed pertussis after three infant doses, with follow-up studies suggesting sustained efficacy to age 6 years.[13, 54, 55] In a Swiss study, effectiveness against pertussis hospitalisation increased with each consecutive primary dose in infants from age 2.5 months to 2 years from 42.1 percent (95% CI: 11.3–62.6) after the first dose, 83.9 percent (70.2–92.1) after the second then 98.2 percent (96.1–99.3) to 100 percent (97.9–100) after the third and fourth doses.[56]
While effective, observational data from Australia found that acellular pertussis vaccines may be less effective than the best-performing whole-cell vaccines in preventing whooping cough.[57, 58] However, the quality of the whole cell vaccine varied between countries and a Canadian study found it to be less effective than the acellular vaccine.[59]
Age-appropriate pertussis vaccination in the US was shown to reduce the severity of symptoms and complications of the disease, including 60 percent reduction in the odds of severe disease (seizure, encephalopathy, pneumonia and/or hospitalisation) in children aged 7 months to 6 years, and 30 percent reduction in post-tussive vomiting in those aged 19 months to 64 years.[60]
Duration of protection
Protection against pertussis begins to wane within several years of completion of a three-dose primary and two-dose booster immunisation series. The US has a pertussis immunisation schedule that includes three doses of acellular vaccine during infancy and booster doses at 15 to 18 months and 4 to 6 years.[61] The risk of pertussis increases in the six years after receipt of the fifth dose of this series, indicating a waning in vaccine-induced immunity over this time interval.
A decline in effectiveness was seen in children more distant from the last DTaP dose, by 27 percent per year on average.[62] Waning effectiveness is more rapid following the adolescent booster at around 35 percent per year.[63] In children, vaccine was 80 percent (95% CI: 71–86) effective against pertussis from two weeks to a year following vaccination, 84 percent (77–89) after 1–3 years, declining to 62 percent (42–75) after 4–7 years and to 41 percent (0–66) at eight or more years after vaccination.[64] A meta-analysis estimated that only 10 percent of those vaccinated with five doses of DTaP would be immune to pertussis 8.5 years after their last DTaP dose.[65]
Antibodies to pertussis toxoid, filamentous hemagglutinin and pertactin have been shown to persist five years after receipt of Tdap (Boostrix) in a study of Australian adults aged 18 years and older.[66 (external link)] However, the duration of protection is unknown.
16.4.3. Transport, storage and handling
16.4.3. Transport, storage and handling
Transport according to the National Standards for Vaccine Storage and Transportation for Immunisation Providers 2017 (2nd edition).
Store at +2°C to +8°C. Do not freeze. DTaP-IPV-HepB/Hib should be stored in the dark.
DTaP-IPV-HepB/Hib (Infanrix-hexa) must be reconstituted by adding the entire contents of the supplied container of the DTaP‑IPV-HepB vaccine to the vial containing the Hib-PRP pellet. After adding the vaccine to the pellet, the mixture should be shaken until the pellet is completely dissolved. Use the reconstituted vaccine as soon as possible. If storage is necessary, the reconstituted vaccine may be kept for up to eight hours at 21°C.
16.4.4. Dosage and administration
16.4.4. Dosage and administration
The dose of DTaP-IPV-HepB/Hib, DTaP-IPV and Tdap is 0.5 mL, administered by intramuscular injection (see section 2.2.3 (external link)).
Co-administration with other vaccines
DTaP-IPV-HepB/Hib, DTaP-IPV and Tdap can be administered simultaneously (at separate sites) with other vaccines or IGs.
16.5. Recommended immunisation schedule
Table 16.1: Immunisation schedule for pertussis-containing vaccines (excluding catch-up)
Table 16.1: Immunisation schedule for pertussis-containing vaccines (excluding catch-up)
|
Age |
Vaccine |
Comment |
|---|---|---|
|
Pregnant women: recommended from 16 weeks’ gestation of every pregnancy, preferably in the second trimester (funded when given any time in second or third trimester) |
Tdap |
Booster for mother Passive immunity for infant |
|
6 weeks |
DTaP-IPV-HepB/Hib |
Primary series |
|
3 months |
DTaP-IPV-HepB/Hib |
Primary series |
|
5 months |
DTaP-IPV-HepB/Hib |
Primary series |
|
4 years |
DTaP-IPV |
Booster |
|
11 years |
Tdap |
Booster |
|
45 years (individuals who have not received 4 tetanus vaccinations in their lifetime) |
Tdap |
Booster |
|
From 65 years |
Tdap |
Booster |
16.5.1. Children
16.5.1. Children
A primary course of pertussis vaccine is given as DTaP-IPV-HepB/Hib (Infanrix-hexa) at ages 6 weeks, 3 months and 5 months, followed by a dose of DTaP-IPV (Infanrix-IPV) at age 4 years (Table 16.1 (external link)). A further booster is given at age 11 years (school year 7) as Tdap (Boostrix).
It is important to administer all vaccinations on time. Delays in receipt of infant immunisations significantly increase the risk of infants being hospitalised for severe pertussis.[67]
If a course of immunisation is late or interrupted for any reason, it may be resumed without repeating prior doses (see Appendix 2). The minimum interval between doses is four weeks. A booster dose should be given no earlier than six months after the primary series.
Catch-up immunisation
See Appendix 2 (external link) for detailed catch-up immunisation information.
- DTaP-IPV-HepB/Hib or DTaP-IPV may be used for primary immunisation and boosting of children aged under 10 years.
- Tdap may be used for primary immunisation and boosting of children aged 7 to under 18 years.
Tdap is also funded:
- as a single dose for vaccination of patients from aged 65 years old (if not previously received 65-year-old Td dose, within the last 10 years)
- as single dose for vaccination of patients aged 45 years old who have not had 4 previous tetanus doses
- for vaccination of previously unimmunised or partially immunised patients
- for vaccination prior to planned or revaccination following immunosuppression (see section 16.5.3 (external link))
- for boosting of patients with tetanus-prone wounds (see section 22.5.5 (external link)).
16.5.2. Pregnancy and breastfeeding
16.5.2. Pregnancy and breastfeeding
Pregnant women should receive a dose of Tdap in every pregnancy so that antibodies can pass to the fetus to provide protection from birth (funded when given any time in second or third trimester). It is recommended to be given from 16 weeks’ gestation of every pregnancy, preferably in the second trimester to protect both the mother and her infant from pertussis.[47, 51] Post-partum maternal vaccination may reduce the risk of a mother infecting her baby but does not have the added benefit of providing the baby with passive antibodies (see section 16.4.2 (external link) for details of effectiveness).
Maternal Tdap vaccination has been shown to prevent pertussis disease or reduce severity of the disease and risk of pertussis-related death in very young infants.[68] There is no evidence that Tdap vaccination affects pregnancy outcomes[68, 69, 70] or causes harm to the fetus or neonate.[68, 71]
Tdap vaccines can be given to breastfeeding women, if missed receiving Tdap during pregnancy.[72 (external link)] Ideally, to be given as near to birth of the infant as possible.
16.5.3. Recommended and funded
16.5.3. Recommended and funded
Parents or primary caregivers of infants admitted to neonatal intensive or special care baby units for more than 3 days and whose mothers had not received Tdap at least 14 days prior to birth.
Tdap is also funded:
- as a single dose for vaccination of patients from aged 65 years old (if not previously received 65-year-old Td dose, within the last 10 years)
- as single dose for vaccination of patients aged 45 years old who have not had 4 previous tetanus doses
- for vaccination of previously unimmunised or partially immunised patients
- for vaccination prior to planned or revaccination following immunosuppression (see section 16.5.3)
- for boosting of patients with tetanus-prone wounds (see section 22.5.5).
16.5.4. (Re)vaccination
16.5.4. (Re)vaccination
Pertussis-containing vaccines are funded for vaccination or re-vaccination of eligible patients who have become immunocompromised, as follows. See also sections 4.2 and 4.3.
DTaP-IPV-HepB/Hib (Infanrix-hexa) and DTaP-IPV (Infanrix-IPV)
An additional four doses (as appropriate) of DTaP-IPV-HepB/Hib (for children aged under 10 years) or DTaP-IPV are funded for (re)vaccination of patients:
- post-HSCT or chemotherapy
- pre- or post-splenectomy
- pre- or post-solid organ transplant
- undergoing renal dialysis
- prior to planned or following other severely immunosuppressive regimens.
Up to an additional four doses (as appropriate) of DTaP-IPV-HepB/Hib are funded for children aged 10 years to under 18 years for (re)vaccination of patients post HSCT. This is an off-label use of this vaccine which requires a prescription from an authorised prescriber or the patient’s specialist. In this group, all prior immunity will have been lost and revaccination is equivalent to a primary immunisation schedule. DTaP-IPV-HepB/Hib not recommended for other severely immunocompromised individuals aged 10-18 years that require revaccination as they may have residual immune memory.
Tdap (Boostrix)
An additional four doses (as appropriate) of Tdap (Boostrix) are funded for patients:
- post-HSCT or chemotherapy
- pre- or post-splenectomy
- pre- or post-solid organ transplant
- undergoing renal dialysis
- prior to planned or following other severely immunosuppressive regimens.
A single dose of Tdap is funded for parents or primary caregivers of infants admitted to a neonatal intensive care unit or special care baby unit for more than three days and whose mothers had not received Tdap at least 14 days prior to baby’s birth.
16.5.5. Recommended but not funded
16.5.5. Recommended but not funded
Tdap is recommended but not funded, unless given as prophylaxis for a tetanus-prone wound, for protection against pertussis for:
- professions and students of professions in contact with infants, a booster dose given 5- to 10‑year intervals (depending on employer requirements), for example, lead maternity carers and other health care personnel who work in neonatal units, other clinical settings (such as GPs and practice nurses) and early childhood education services staff (see Table 4.9). Infants with respiratory, cardiac, neurological or other co-morbid conditions are particularly at risk from pertussis.
- household members and other who have regular close contact with a newborn, is recommended to have a booster dose of Tdap if they have not had a dose in the previous 10 years, ideally at least two weeks prior to the birth of the infant. This is particularly important in households where the mother had not received Tdap vaccination in pregnancy more than seven days prior to birth of the infant. Household members aged under 18 years who are unimmunised or incompletely immunised for their age can receive funded pertussis vaccine; see Appendix 2 for catch-up schedules.
- regardless of maternal vaccination history, all caregivers of infants born at less than 32 weeks’ gestation are recommended to receive a single dose of Tdap (see section 4.2.2)
- early childhood workers and students are recommended a Tdap booster dose at 5 to 10-year intervals, although the priority is to ensure all children attending childcare have received age-appropriate vaccination
- adults with a medical condition, not eligible for funded vaccine, who are at increased risk of severe consequences of pertussis (eg, those with chronic respiratory disease).
16.6. Contraindications and precautions
See also section 2.1.3 for pre-vaccination screening guidelines and section 2.1.4 (external link) for general contraindications for all vaccines.
16.6.1. Contraindications
16.6.1. Contraindications
The only contraindication is an immediate severe anaphylactic reaction to the vaccine, or any component of the vaccine, following a previous dose.
16.6.2. Precautions
16.6.2. Precautions
A history of well-controlled seizures in the vaccine recipient or a family history of seizures (febrile or afebrile) or other neurologic disorder is not a contraindication to vaccination against pertussis.[50]
Although previously been considered a precaution, vaccination is recommended for infants with an unstable neurological disorder (eg, poorly controlled epilepsy or deteriorating neurological state) as they may be at high risk of severe pertussis complications.[50 (external link)]
16.7. Potential responses and AEFIs
Unless the specific contraindications and precautions outlined in section 16.6 above are present, practitioners should have no hesitation in advising the administration of acellular pertussis vaccine. Acellular pertussis vaccine has been used in New Zealand since 2000 and is significantly less reactogenic than the whole-cell pertussis vaccine.
16.7.1. DTaP-containing vaccines
16.7.1. DTaP-containing vaccines
DTaP-containing vaccines (eg, DTaP-IPV-HepB/Hib and DTaP-IPV) are generally well tolerated in children,[73] including preterm (24 to 36 weeks’ gestation) and/or low birthweight (820–2,020 g) infants.[74, 75]
Local reactions commonly include pain, redness, swelling and induration at the injection site. Less common reactions include fretfulness, anorexia, vomiting, crying and slight to moderate fever. These local and systemic reactions usually occur within several hours of pertussis immunisation and spontaneously resolve within 48 hours without sequelae.[73]
Local reactions increase with age and additional doses of vaccine. The reaction may be due to some of the other vaccine components, such as aluminium. These reactions are usually minor and only last a day or so.
16.7.2. Tdap vaccine
16.7.2. Tdap vaccine
The adult reduced-concentration Tdap (Boostrix) vaccines have been found to have no safety concerns in those aged 10–64 years and those aged over 65 years.[76, 77, 78, 79] Studies of Tdap in pregnant women have not identified any increased risk of adverse maternal, infant or fetal outcomes.[12, 71, 80, 81, 82]
Local reactions following immunisation of adolescents with Tdap are common but usually mild. They include pain (in 75 percent of recipients), swelling (21 percent) and redness (23 percent) at the injection site.[83] Potential systemic reactions following immunisation of adolescents with Tdap include fever >38°C (5 percent), headache (16 percent), fatigue (14 percent) and gastrointestinal symptoms (10 percent).[83 (external link)]
16.7.3. Major adverse events associated with pertussis-containing vaccines
16.7.3. Major adverse events associated with pertussis-containing vaccines
The incidence of major adverse events following primary pertussis immunisation is summarised in Table 16.2.
Table 16.2: Incidence of major adverse reactions following acellular pertussis vaccines (based on clinical trial data for DTaP vaccines)
|
Event following immunisation |
Timing |
Incidence per 100,000 doses |
|---|---|---|
|
High fever >38°C |
0–2 days |
36 |
|
Persistent (>3 hours) inconsolable screaming |
0–24 hours |
44 |
|
Seizures |
0–2 days |
7 |
|
Hypotonic-hyporesponsive episode |
0–2 days |
0–47a |
|
Anaphylaxis |
0–1 hour |
Very rare |
|
a. Across clinical trials of multiple licensed DTaP formulations Source: Edwards KM, Decker MD. 2018. Pertussis vaccines. In: Plotkin S, Orenstein W, Offit P, et al (eds) Plotkin’s Vaccines (7th edition). Elsevier. |
||
Parents should be alerted to the small but defined risk of extensive limb swelling to the injected thigh or upper arm, particularly following the fourth and fifth DTaP dose. This transient, usually painless and benign swelling occurs in 2–3 percent of children.[73] Resolution occurs without sequelae and it is not a contraindication for further pertussis vaccine doses.[72, 84]
Neither a hypotonic-hyporesponsive episode nor seizures are associated with long-term consequences for the child (see section 2.3.3).[85, 86, 87] Children who have febrile seizures after pertussis immunisation do not have an increased risk of subsequent seizures or neurodevelopmental disability.[88] It is safe to give acellular pertussis vaccine after a hypotonic-hyporesponsive episode has occurred following a previous dose.[89] A significant decrease of 60–67 percent in hypotonic-hyporesponsive episodes was observed in Canada following the switch from whole cell to acellular pertussis vaccines.[90]
16.8. Public health measures
16.8.1. Notification
16.8.1. Notification
It is a legal requirement that all cases of pertussis be notified immediately on suspicion to the local medical officer of health.
For detailed information about public health control measures, see the Communicable Disease Control Manual pertussis chapter.
A suspected pertussis case can be confirmed if a clinically compatible illness is laboratory-confirmed or is epidemiologically linked to a confirmed case. Because transmission is by aerosolised droplets, health care personnel looking after pertussis cases should be vaccinated and wear a mask.
16.8.2. Management of contacts
16.8.2. Management of contacts
The local medical officer of health will advise on the management of contacts. For more details on control measures, see the Communicable Disease Control Manual pertussis chapter.
16.8.3. Improving pertussis control
16.8.3. Improving pertussis control
The goal of the pertussis immunisation programme is to protect those most at risk of developing severe disease; that is, infants in the first year of life. Two key strategies for reducing the burden of disease in infants are the administration of Tdap vaccination during pregnancy and on-time infant immunisation. Vaccination during pregnancy is recommended and funded for women from the second trimester, preferably from 16 weeks’ gestation (see section 16.5.2). This is the most effective way to protect young infants. More complete and timely delivery of the current infant immunisation schedule would reduce the infant pertussis disease burden in older infants.[67] It is important that all children attending early childhood services are fully vaccinated for their age.
Data on the protective effects of indirect strategies is currently incomplete. ‘Cocoon strategy’ is the term used to describe the protection of infants by immunising those who are potential sources of B. pertussis.[91] Three identified target groups who have the most contact with young and vulnerable infants are (1) new mothers who have not had recent immunisation, family and close contacts of newborns; (2) health care workers; and (3) early childhood workers. Some protection may be provided to infants by cocoon immunisation of parents and other potential household members post-partum, may be pertinent in some circumstances where maternal vaccination did not occur, such as preterm birth, and infants in neonatal intensive care.[92]
Health care workers in particular are at increased risk of pertussis and can transmit pertussis to other health care workers and to patients.[93] Outbreaks in maternity wards, neonatal units and outpatient settings have been described.[94] Fatalities occur as a result of such nosocomial spread.[95 (external link)]
Mass immunisation cannot be used to control an established community outbreak, although action to update age-appropriate vaccination in institutional settings (schools and early childhood services for staff and students) is appropriate. When an outbreak occurs, individual immunisation status should be checked, and any missing doses given. Vaccination in pregnancy is particularly important to protect the most vulnerable, young infants.
16.9. Variations from the vaccine data sheets
To reduce the number of injections required and to provide a higher antigen dose than Tdap, DTaP-IPV-HepB/Hib is recommended and funded for the revaccination of children aged 10 to <18 years post-haematopoietic stem cell transplantation. This use requires a prescription from an authorised prescriber.
The data sheets for DTaP-IPV-HepB/Hib, DTaP-IPV and Tdap (Boostrix) state that these vaccines are contraindicated in children with encephalopathy of unknown aetiology or with neurologic complications occurring within seven days following a vaccine dose. Health NZ recommends that the only contraindication is a history of anaphylaxis to a previous dose or to any of the vaccine components (see section 16.6.1). The risks and benefits of withholding vaccination until the clinical situation has stabilised should be considered on an individual basis (see section 16.6.2).
References
References
References
- Fine P, Mulholland K, Scott J,Edmunds W. 2018. Community Protection, in Plotkin's Vaccines (7th edition), Plotkin S, Orenstein W, Offit P, and Edwards K (eds). Elsevier: Philadelphia, US.
- McGovern MC ,Smith MB. Causes of apparent life threatening events in infants: a systematic review. Archives of Disease in Childhood, 2004. 89(11): p. 1043-8.
- Harnden A, Grant C, Harrison T, et al. Whooping cough in school age children with persistent cough: prospective cohort study in primary care. BMJ, 2006. 333(7560): p. 174-7.
- Moore A, Ashdown HF, Shinkins B, et al. Clinical characteristics of pertussis-associated cough in adults and children: A diagnostic systematic review and meta-analysis. Chest, 2017. 152(2): p. 353-367.
- Wirsing von König C-H, Halperin S, Riffelmann M,Guiso N. Pertussis of adults and infants. Lancet Infectious Diseases, 2002. 2(12): p. 744-50.
- Ebell MH, Marchello C ,Callahan M. Clinical diagnosis of Bordetella pertussis infection: a systematic review. Journal of the American Board of Family Medicine, 2017. 30(3): p. 308-319.
- Robertson PW, Goldberg H, Jarvie BH, et al. Bordetella pertussis infection: a cause of persistent cough in adults. Medical Journal of Australia, 1987. 146(10): p. 522-5.
- Senzilet LD, Halperin SA, Spika JS, et al. Pertussis is a frequent cause of prolonged cough illness in adults and adolescents. Clinical Infectious Diseases, 2001. 32(12): p. 1691-7.
- Gilberg S, Njamkepo E, Du Chatelet IP, et al. Evidence of Bordetella pertussis infection in adults presenting with persistent cough in a french area with very high whole-cell vaccine coverage. Journal of Infectious Diseases, 2002. 186(3): p. 415-8.
- Philipson K, Goodyear-Smith F, Grant CC, et al. When is acute persistent cough in school-age children and adults whooping cough? A prospective case series study. British Journal of General Practice, 2013. 63(613): p. e573-9.
- Surridge J, Segedin ER ,Grant CC. Pertussis requiring intensive care. Archives of Disease in Childhood, 2007. 92(11): p. 970-5.
- Cherry JD. The epidemiology of pertussis: a comparison of the epidemiology of the disease pertussis with the epidemiology of Bordetella pertussis infection. Pediatrics, 2005. 115(5): p. 1422-7.
- Edwards KM ,Decker MD. 2018. Pertussis vaccines, in Vaccines 7th Edition, Plotkin S, Orenstein W,Offit P (eds). Elsevier
- Craig R, Kunkel E, Crowcroft NS, et al. Asymptomatic infection and transmission of pertussis in households: a systematic review. Clinical Infectious Diseases, 2019.
- Leong RNF, Wood JG, Turner RM,Newall AT. Estimating seasonal variation in Australian pertussis notifications from 1991 to 2016: evidence of spring to summer peaks. Epidemiology and Infection, 2019. 147: p. e155.
- Gordon JE ,Hood RI. Whooping cough and its epidemiological anomalies. American Journal of the Medical Sciences, 1951. 222(3): p. 333-61.
- Haberling DL, Holman RC, Paddock CD,Murphy TV. Infant and maternal risk factors for pertussis-related infant mortality in the United States, 1999 to 2004. Pediatric Infectious Disease Journal, 2009. 28(3): p. 194-8.
- Sutter RW ,Cochi SL. Pertussis hospitalizations and mortality in the United States, 1985-1988. Evaluation of the completeness of national reporting. JAMA, 1992. 267(3): p. 386-91.
- Crowcroft NS ,Pebody RG. Recent developments in pertussis. Lancet, 2006. 367(9526): p. 1926-36.
- Shaikh R, Guris D, Strebel PM,Wharton M. Underreporting of pertussis deaths in the United States: need for improved surveillance. Pediatrics, 1998. 101(2): p. 323.
- Crowcroft NS, Andrews N, Rooney C, et al. Deaths from pertussis are underestimated in England. Archives of Disease in Childhood, 2002. 86(5): p. 336-8.
- Kandeil W, Atanasov P, Avramioti D, et al. The burden of pertussis in older adults: what is the role of vaccination? A systematic literature review. Expert Rev Vaccines, 2019. 18(5): p. 439-455.
- Karki S, McIntyre P, Newall AT, et al. Risk factors for pertussis hospitalizations in Australians aged 45 years and over: A population based nested case-control study. Vaccine, 2015. 33(42): p. 5647-5653.
- Mikelova LK, Halperin SA, Scheifele D, et al. Predictors of death in infants hospitalized with pertussis: a case-control study of 16 pertussis deaths in Canada. Journal of Pediatrics, 2003. 143(5): p. 576-81.
- Winter K, Zipprich J, Harriman K, et al. Risk factors associated with infant deaths from pertussis: a case-control study. Clinical Infectious Diseases, 2015. 61(7): p. 1099-106.
- Cherry JD. The prevention of severe pertussis and pertussis deaths in young infants. Expert Rev Vaccines, 2019. 18(3): p. 205-208.
- Joo I, Epidemiology of pertussis in Hungary, in Developments in Biological Standardization. 1991. p. 357–9.
- Kimura M ,Kuno-Sakai H. Developments in pertussis immunisation in Japan. Lancet, 1990. 336(8706): p. 30-2.
- Miller E, Vurdien JE ,White JM. The epidemiology of pertussis in England and Wales. Communicable Disease Report. CDR Review, 1992. 2(13): p. R152-4.
- Domenech de Celles M, Magpantay FMG, King AA,Rohani P. The impact of past vaccination coverage and immunity on pertussis resurgence. Science Translational Medicine, 2018. 10(434).
- Farizo KM, Cochi SL, Zell ER, et al. Epidemiological features of pertussis in the United States, 1980-1989. Clinical Infectious Diseases, 1992. 14(3): p. 708-19.
- Provenzano RW, Wetterlow LH ,Ipsen J. Pertussis immunization in pediatric practice and in public health. New England Journal of Medicine, 1959. 261(10): p. 473-8.
- Guris D, Strebel PM, Bardenheier B, et al. Changing epidemiology of pertussis in the United States: increasing reported incidence among adolescents and adults, 1990–1996. Clinical Infectious Diseases, 1999. 28(6): p. 1230–7.
- Zerbo O, Bartlett J, Goddard K, et al. Acellular Pertussis Vaccine Effectiveness Over Time. Pediatrics, 2019. 144(1).
- Ranganathan S, Tasker R, Booy R, et al. Pertussis is increasing in unimmunized infants: is a change in policy needed? Archives of Disease in Childhood, 1999. 80(3): p. 297-9.
- Tanaka M, Vitek CR, Pascual FB, et al. Trends in pertussis among infants in the United States, 1980-1999. JAMA, 2003. 290(22): p. 2968-75.
- Broutin H, Guegan JF, Elguero E, et al. Large-scale comparative analysis of pertussis population dynamics: periodicity, synchrony, and impact of vaccination. American Journal of Epidemiology, 2005. 161(12): p. 1159-67.
- Institute of Environmental Science and Research Ltd. 2016. Notifiable Diseases in New Zealand: Annual Report 2015 (ed.), Porirua, New Zealand: The Institute of Science and Environmental Research Ltd. URL: https://surv.esr.cri.nz/PDF_surveillance/AnnualRpt/AnnualSurv/2015/2015AnnualReportFinal.pdf (external link) (accessed 3 July 2020)
- Institute of Environmental Science and Research. 2019 Pertussis Report May 2019. Porirua, Wellington. URL: https://surv.esr.cri.nz/PDF_surveillance/PertussisRpt/2019/PertussisReportMay2019.pdf (external link). (accessed 3 July 2020)
- Ganeshalingham A, Reed P, Grant C, et al. Hospital costs of Bordetella pertussis in New Zealand children. New Zealand Medical Journal, 2016. 129(1445): p. 75-82.
- Elliott E, McIntyre P, Ridley G, et al. National study of infants hospitalized with pertussis in the acellular vaccine era. Pediatric Infectious Disease Journal, 2004. 23(3): p. 246-52.
- Cortese MM, Baughman AL, Zhang R, et al. Pertussis hospitalizations among infants in the United States, 1993 to 2004. Pediatrics, 2008. 121(3): p. 484-92.
- Grant CC. Recent indication of progress in pertussis hospitalisation rates in NZ. Australian and New Zealand Journal of Public Health, 2012. 36(4): p. 398-398.
- Simpson J, Duncanson M, Oben G, et al. 2016 The Health Status of Children and Young People in New Zealand 2015.: Dunedin. URL: https://www.otago.ac.nz/nzcyes/reports-by-category/reports-by-year/index.html (external link). (accessed 3 July 2020)
- Dhillon S. DTPa-HBV-IPV/Hib vaccine (Infanrix hexa): a review of its use as primary and booster vaccination. Drugs, 2010. 70(8): p. 1021-58.
- Zepp F, Schmitt HJ, Cleerbout J, et al. Review of 8 years of experience with Infanrix hexa (DTPa-HBV-IPV/Hib hexavalent vaccine). Expert Rev Vaccines, 2009. 8(6): p. 663-78.
- Amirthalingam G, Campbell H, Ribeiro S, et al. Sustained effectiveness of the maternal pertussis immunization program in England 3 years following introduction. Clinical Infectious Diseases, 2016. 63(suppl 4): p. S236-S243.
- Amirthalingam G, Andrews N, Campbell H, et al. Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet, 2014. 384(9953): p. 1521-8.
- Winter K, Nickell S, Powell M,Harriman K. Effectiveness of prenatal versus postpartum tetanus, diphtheria, and acellular pertussis vaccination in preventing infant pertussis. Clinical Infectious Diseases, 2017. 64(1): p. 3-8.
- Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR: Recommendations and Reports, 2018. 67(2): p. 1-44.
- Eberhardt CS, Blanchard-Rohner G, Lemaitre B, et al. Maternal immunization earlier in pregnancy maximizes antibody transfer and expected infant seropositivity against pertussis. Clinical Infectious Diseases, 2016. 62(7): p. 829-836.
- Eberhardt CS, Blanchard-Rohner G, Lemaitre B, et al. Pertussis antibody transfer to preterm neonates after second- versus third-trimester maternal immunization. Clinical Infectious Diseases, 2017. 64(8): p. 1129-1132.
- Kent A, Ladhani SN, Andrews NJ, et al. Pertussis antibody concentrations in infants born prematurely to mothers vaccinated in pregnancy. Pediatrics, 2016. 138(1): p. 07.
- Greco D, Salmaso S, Mastrantonio P, et al. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. Progetto Pertosse Working Group. New England Journal of Medicine, 1996. 334(6): p. 341-8.
- Gustafsson L, Hessel L, Storsaeter J,Olin P. Long-term follow-up of Swedish children vaccinated with acellular pertussis vaccines at 3, 5, and 12 months of age indicates the need for a booster dose at 5 to 7 years of age. Pediatrics, 2006. 118(3): p. 978-84.
- Mack I, Erlanger TE, Lang P, et al. Dose-dependent effectiveness of acellular pertussis vaccine in infants: A population-based case-control study. Vaccine, 2019.
- Sheridan SL, Ware RS, Grimwood K,Lambert SB. Number and order of whole cell pertussis vaccines in infancy and disease protection. JAMA, 2012. 308(5): p. 454-6.
- Sheridan SL, Ware RS, Grimwood K,Lambert SB. Reduced risk of pertussis in whole-cell compared to acellular vaccine recipients is not confounded by age or receipt of booster-doses. Vaccine, 2015. 33(39): p. 5027-30.
- Wilkinson K, Righolt CH, Kwong JC, et al. A nested case-control study measuring pertussis vaccine effectiveness and duration of protection in Manitoba, Canada, 1992-2015: A Canadian Immunization Research Network Study. Vaccine, 2019. 37(48): p. 7132-7137.
- McNamara LA, Skoff T, Faulkner A, et al. Reduced severity of pertussis in persons with age-appropriate pertussis vaccination – United States, 2010–2012. Clinical Infectious Diseases, 2017. 65(5): p. 811-818.
- Tartof SY, Lewis M, Kenyon C, et al. Waning immunity to pertussis following 5 doses of DTaP. Pediatrics, 2013. 131(4): p. e1047-52.
- Klein NP, Bartlett J, Fireman B, et al. Waning protection following 5 doses of a 3-component diphtheria, tetanus, and acellular pertussis vaccine. Vaccine, 2017. 35(26): p. 3395-3400.
- Klein NP, Bartlett J, Fireman B,Baxter R. Waning Tdap Effectiveness in Adolescents. Pediatrics, 2016. 137(3): p. e20153326.
- Schwartz KL, Kwong JC, Deeks SL, et al. Effectiveness of pertussis vaccination and duration of immunity. CMAJ: Canadian Medical Association Journal, 2016. 188(16): p. E399-E406.
- McGirr A ,Fisman DN. Duration of pertussis immunity after DTaP immunization: a meta-analysis. Pediatrics, 2015. 135(2): p. 331-43.
- McIntyre PB, Burgess MA, Egan A, et al. Booster vaccination of adults with reduced-antigen-content diphtheria, Tetanus and pertussis vaccine: immunogenicity 5 years post-vaccination. Vaccine, 2009. 27(7): p. 1062-6.
- Grant CC, Roberts M, Scragg R, et al. Delayed immunisation and risk of pertussis in infants: unmatched case-control study. BMJ, 2003. 326(7394): p. 852-3.
- Campbell H, Gupta S, Dolan GP, et al. Review of vaccination in pregnancy to prevent pertussis in early infancy. Journal of Medical Microbiology, 2018. 67(10): p. 1426-1456.
- Griffin JB, Yu L, Watson D, et al. Pertussis Immunisation in Pregnancy Safety (PIPS) Study: A retrospective cohort study of safety outcomes in pregnant women vaccinated with Tdap vaccine. Vaccine, 2018. 36(34): p. 5173-5179.
- McHugh L, Marshall HS, Perrett KP, et al. The safety of influenza and pertussis vaccination in pregnancy in a cohort of australian mother-infant pairs, 2012-2015: The FluMum Study. Clinical Infectious Diseases, 2019. 68(3): p. 402-408.
- Petousis-Harris H, Jiang Y, Yu L, et al. A retrospective cohort study of safety outcomes in New Zealand infants exposed to Tdap vaccine in utero. Vaccines (Basel), 2019. 7(4).
- Australian Technical Advisory Group on Immunisation. 2018. Australian Immunisation Handbook (ed.), Canberra: Australian Government Department of Health. URL: https://immunisationhandbook.health.gov.au/ (external link) (accessed October 2019)
- American Academy of Pediatrics. 2018. Pertussis (Whooping Cough). in Red Book: 2018 Report of the Committee on Infectious Diseases, Committee on Infectious Diseases, Kimberlin D, Brady M, et al. (eds). Elk Grove Village, IL. URL: https://redbook.solutions.aap.org/redbook.aspx (external link). (accessed 3 July 2020)
- Omeñaca F, Garcia-Sicilia J, García-Corbeira P, et al. Response of preterm newborns to immunization with a hexavalent diphtheria-tetanus-acellular pertussis-hepatitis B virus-inactivated polio and Haemophilus influenzae type b vaccine: first experiences and solutions to a serious and sensitive issue. Pediatrics, 2005. 116(6): p. 1292-8.
- Lyseng-Williamson KA ,Dhillon S. DTPa-HBV-IPV/Hib vaccine (Infanrix hexa): a guide to its use in infants. Paediatric Drugs, 2012. 14(5): p. 337-43.
- Jackson LA, Yu O, Belongia EA, et al. Frequency of medically attended adverse events following tetanus and diphtheria toxoid vaccine in adolescents and young adults: a Vaccine Safety Datalink study. BMC Infectious Diseases, 2009. 9(165): p. 165.
- Yih WK, Nordin JD, Kulldorff M, et al. An assessment of the safety of adolescent and adult tetanus-diphtheria-acellular pertussis (Tdap) vaccine, using active surveillance for adverse events in the Vaccine Safety Datalink. Vaccine, 2009. 27(32): p. 4257-62.
- Moro PL, Yue X, Lewis P, et al. Adverse events after tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine administered to adults 65 years of age and older reported to the Vaccine Adverse Event Reporting System (VAERS), 2005-2010. Vaccine, 2011. 29(50): p. 9404-8.
- Baxter R, Hansen J, Timbol J, et al. Post-licensure safety surveillance study of routine use of tetanus toxoid, reduced diphtheria toxoid and 5-component acellular pertussis vaccine. Human Vaccines & Immunotherapeutics, 2016. 12(11): p. 2742-2748.
- Zheteyeva YA, Moro PL, Tepper NK, et al. Adverse event reports after tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines in pregnant women. American Journal of Obstetrics and Gynecology, 2012. 207(1): p. 59 e1-7.
- Donegan K, King B ,Bryan P. Safety of pertussis vaccination in pregnant women in UK: observational study. BMJ, 2014. 349(11 July): p. g4219.
- Petousis-Harris H, Walls T, Watson D, et al. Safety of Tdap vaccine in pregnant women: an observational study. BMJ Open, 2016. 6(4): p. e010911.
- Centers for Disease Control and Prevention. 2006. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report. 55(RR-3): p. 1–44. https://www.cdc.gov/mmwr/pdf/rr/rr5503.pdf (external link) (accessed 3 July 2020)
- Southern J, Waight PA, Andrews N,Miller E. Extensive swelling of the limb and systemic symptoms after a fourth dose of acellular pertussis containing vaccines in England in children aged 3-6years. Vaccine, 2017. 35(4): p. 619-625.
- Hirtz DG, Nelson KB ,Ellenberg JH. Seizures following childhood immunizations. Journal of Pediatrics, 1983. 102(1): p. 14-8.
- Baraff LJ, Shields WD, Beckwith L, et al. Infants and children with convulsions and hypotonic-hyporesponsive episodes following diphtheria-tetanus-pertussis immunization: follow-up evaluation. Pediatrics, 1988. 81(6): p. 789-94.
- Braun MM, Terracciano G, Salive ME, et al. Report of a US public health service workshop on hypotonic-hyporesponsive episode (HHE) after pertussis immunization. Pediatrics, 1998. 102(5): p. E52.
- Barlow WE, Davis RL, Glasser JW, et al. The risk of seizures after receipt of whole-cell pertussis or measles, mumps, and rubella vaccine. New England Journal of Medicine, 2001. 345(9): p. 656-61.
- Goodwin H, Nash M, Gold M, et al. Vaccination of children following a previous hypotonic-hyporesponsive episode. Journal of Paediatrics and Child Health, 1999. 35(6): p. 549-52.
- Le Saux N, Barrowman NJ, Moore DL, et al. Decrease in hospital admissions for febrile seizures and reports of hypotonic-hyporesponsive episodes presenting to hospital emergency departments since switching to acellular pertussis vaccine in Canada: a report from IMPACT. Pediatrics, 2003. 112(5): p. e348-e348.
- McIntyre P ,Wood N. Pertussis in early infancy: disease burden and preventive strategies. Current Opinion in Infectious Diseases, 2009. 22(3): p. 215-23.
- Rowe SL, Tay EL, Franklin LJ, et al. Effectiveness of parental cocooning as a vaccination strategy to prevent pertussis infection in infants: A case-control study. Vaccine, 2018. 36(15): p. 2012-2019.
- De Serres G, Shadmani R, Duval B, et al. Morbidity of pertussis in adolescents and adults. Journal of Infectious Diseases, 2000. 182(1): p. 174-9.
- Centers for Disease Control and Prevention. 2008. Prevention of pertussis, tetanus, and diphtheria among pregnant and postpartum women and their infants: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report: Recommendations and Reports. 57(RR-4): p. 1–51. https://www.cdc.gov/mmwr/PDF/rr/rr5704.pdf (external link) (accessed 3 July 2020)
- Bonacorsi S, Farnoux C, Bidet P, et al. Treatment failure of nosocomial pertussis infection in a very-low-birth-weight neonate. Journal of Clinical Microbiology, 2006. 44(10): p. 3830-2.