On this page
Key information
|
Mode of transmission |
Inhalation of airborne droplets produced by people with pulmonary or laryngeal tuberculosis (TB). People with latent TB infection and non-pulmonary TB disease are not infectious. |
|---|---|
|
Incubation period |
Between 2 and 10 weeks from infection to primary lesion or significant tuberculin skin test (Mantoux) reaction. |
|
Period of communicability |
May be years with untreated pulmonary TB. See the Guidelines for Tuberculosis Control in New Zealand 2019 (external link) (or current edition) – see section 23.5.3. |
|
Burden of disease |
Disseminated and meningeal TB are more common in very young children. The immunocompromised individuals, particularly HIV-infected, are more at risk of disease and complications. In New Zealand, TB incidence is highest in those born in high prevalence countries. |
|
Funded vaccine |
Bacillus Calmette-Guérin (BCG) Vaccine SSI. |
|
Dose, presentation and route |
Vaccine can only be administered intradermally by an authorised vaccinator with BCG endorsement. Live attenuated vaccine, which must be reconstituted. |
|
Funded vaccine indications and recommended schedule |
Neonatal BCG vaccine should be offered to infants at increased risk of TB, as defined in section 23.5.2. (See section 23.5.2 for countries with a TB rate ≥40 per 100,000.) |
|
Contraindications |
Individuals with primary or secondary immunocompromise, including:
Individuals with generalised infected skin conditions. |
|
Potential responses |
A local reaction develops in 90–95% of those vaccinated with BCG, which may scar within 3 months. A minor degree of adenitis is normal, not a complication. Suppurative adenitis may take months to resolve; usually no treatment is required. |
|
Public health measures |
All cases of active TB must be notified to the local medical officer of health. (see section 23.8) |
|
Post-exposure prophylaxis |
Vaccine prophylaxis is only applicable for young children. |
23.1. Bacteriology
Human tuberculosis (TB) is caused by infection with Mycobacterium tuberculosis or Mycobacterium bovis.
23.2. Clinical features
M. tuberculosis or M. bovis infection most commonly causes disease in the lungs, but any part of the body can be affected.
The initial infection with M. tuberculosis usually goes unnoticed. Early infections can be cleared, progress rapidly to primary TB, or be contained in a latent phase (LTBI): see Figure 23.1.
Primary TB occurs most commonly in young children aged under 5 years, individuals with immunocompromise and those infected by particularly transmissible isolates of TB.
Latent TB infection has no symptoms and is diagnosed by a positive tuberculin skin test or interferon gamma release assay after the exclusion of active TB. Latent infection progressing to active TB is also called reactivation TB.
The lifetime risk for infected people progressing from this latent phase to active TB disease is on average 5-10 percent, usually within the first five years of initial infection. This risk is strongly affected by infecting dose, the age of the person, the presence of healed lesions on chest X-ray, and particularly, immunocompromise.[1, 2, 3, 4]
The time from infection to clinical manifestations of primary TB varies, from one to six months after infection. Reactivation TB can occur at any time thereafter, even decades after infection. The most common site of infection is the lung (pulmonary TB), where TB infection classically causes an asymmetrical pulmonary infiltrate. The ‘classic’ TB pathology of caseation, cavity formation and fibrosis occurs late and in a minority of cases. Young children with active TB disease may be asymptomatic or present with symptoms of fever, lassitude and failure to thrive. Older children and adults with active TB disease may present with symptoms of anorexia, fatigue, weight loss, chills, night sweats, cough, haemoptysis and chest pain.
Any organ can be affected by extrapulmonary TB, causing meningitis, pleurisy, pericarditis, bone or joint infection, renal infection, gastrointestinal tract infection, peritonitis or lymphadenitis, or disseminating via the bloodstream and affecting multiple organs (disseminated TB). Disseminated and meningeal TB are more common in very young children. Immunocompromise, like HIV, is associated with higher rates of disseminated TB and less specific clinical features.[5]
Figure 23.1: Stages in the natural history of tuberculosis
Figure 23.1: Stages in the natural history of tuberculosis
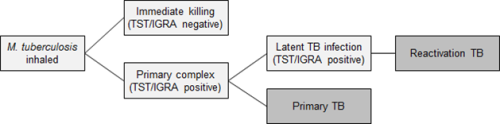
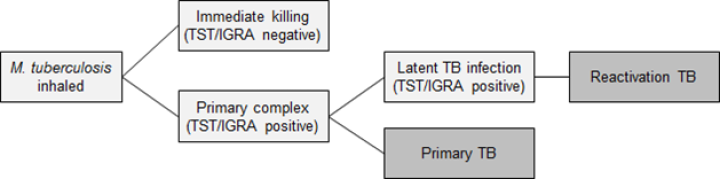
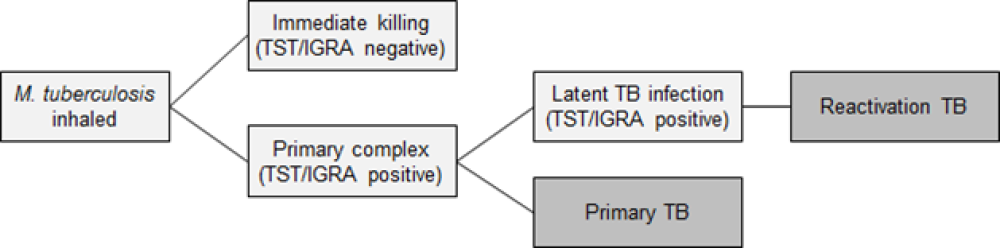
| Key: TST = tuberculin skin test; IGRA = interferon gamma release assay; TB = tuberculosis. |
23.3. Epidemiology
23.3.1. Global epidemiology
23.3.1. Global epidemiology
Around a quarter of the global population is estimated to have been infected with TB bacteria. Worldwide, the incidence of TB has increased, reversing the downward trend observed prior to 2020.[6] Of those infected, about 5–10 percent will develop symptoms and progress to TB disease. The estimated TB incidence rate (new cases per 100,000 population per year) increased by 1.9 percent from 2020 to 2021, and a further 1.9 percent from 2021 to 2022. Disruptions in TB diagnosis and treatment at the start of the COVID-19 pandemic during 2020 led to increases in new cases reporting and TB incidence during the subsequent years. TB remained the world’s second leading cause of death from a single infectious agent in 2022, after COVID-19, with estimated 1.3 million deaths. An estimated 10.6 million people worldwide were diagnosed with TB in 2022, which translates to 133 new cases per 100,000 population. Of these cases, 6.3 percent were among individuals living with HIV.[6]
Most TB cases were reported in the WHO regions of South-East Asia (46 percent), Africa (23 percent), and the Western Pacific (18 percent), with smaller proportions in the Eastern Mediterranean (8.1 percent), the Americas (3.1 percent), and Europe (2.2 percent). There has been an increasing burden of multidrug-resistant TB (defined as resistance to at least isoniazid and rifampicin) with an estimated 410,000 new cases of multidrug-resistant TB in 2022.[6, 7]
In low-burden countries, such as New Zealand, the peak age for TB is in older adults, reflecting their exposure to TB in the past when incidence was higher. In high-burden countries TB is most common in children and young adults. The risk of TB in people who emigrate from high-burden countries is proportionate to the incidence in their country of origin.[8]
23.3.2. New Zealand epidemiology
23.3.2. New Zealand epidemiology
For detailed TB information, see the notifiable disease dashboard at PHF Science (formerly ESR) (external link).
Notification rates and risk factors
TB remains one of the most common notifiable infectious diseases in New Zealand. Cases of TB declined substantially between 1980 and 2007, but they have remained relatively stable since then.[9]
In 2023, there were 317 notifications for TB (notification rate of 6.1 per 100,000), which was higher than 2022 (5.2 per 100,000) (PHF Science, 24 September 2024). Notification rates were highest in those aged 20–29 years (8.5 per 100,000), 40–49 years (8.4 per 100,000) and 30–39 years (8.0 per 100,000), with similar rates in males and females (6.1 and 6.0 per 100,000, respectively).
Asian ethnic groups had the highest notification rate in 2023 (28.2 per 100,000, 224 cases), followed by Middle Eastern/Latin American/African (15.8 per 100,000, 12 cases) and Pacific peoples (13.1 per 100,000, 46 cases). Of the 304 new cases recorded in 2023 with TB risk factors, 267 (87.8 percent) were born overseas (PHF Science, 24 September 2024). The highest disease rate was among those born in Southern and Central Asia (68.2 per 100,000), followed by those born in South-East Asia (63.4 per 100,000), and the Pacific Islands (20.4 per 100,000). The most reported countries of birth were India (92.2 percent) and the Philippines (77.4 percent). Of the 82 percent of new TB cases in 2023 with date of arrival recorded, the median interval between arrival and the TB notification ranged from 0 to 62 years (mean 9 years and median 6 years) (PHF Science, 24 September 2024).
The notification rate was considerably lower for Māori (2.0 per 100,000, 17 cases) and European/ Other (0.5 per 100,000, 15 cases) groups (PHF Science, 24 September 2024). There was substantial regional variation in TB notification rates: the highest rates were in Auckland (11.2 per 100,000), Counties Manukau (10.7 per 100,000), Whanganui (7.1 per 100,000) and Capital and Coast (6.4 per 100,000).
Three children aged under 5 years were notified with TB in 2023 (PHF Science, 24 September 2024). The children were born in New Zealand and not vaccinated with BCG.
Multidrug-resistant TB
Multidrug-resistant TB is rare but does occur in New Zealand. Over 10 years (2012-2021), a total of 39 cases were multidrug resistant (annual average rate of 1.6 percent).(PHF Science, 24 September 2024) All these cases were born and assumed to have acquired the infection overseas: 87.2 percent were born in an Asian country. [10]
23.4. Vaccine
Note: Depending on world supply, BCG vaccine may not be available in New Zealand.
BCG vaccine types vary widely, with different strains. The incidence of side-effects with BCG vaccination differs between strains that are considered more reactogenic (ie, those that elicit stronger immune responses in animal models) and strains that are considered less reactogenic.[11 (external link)] The more reactogenic strains have also been associated with a higher rate of lymphadenitis and osteitis, especially among neonates. Reducing the vaccination dosage for the more reactogenic strains also reduces the incidence of lymphadenitis.
23.4.1. Licensed vaccine
23.4.1. Licensed vaccine
BCG Vaccine SSI (Seqirus (NZ) Ltd) is a live attenuated vaccine, containing the less reactogenic Danish 1331 strain of M. bovis. The 0.1 mL dose for children aged 12 months and older contains 2–8 × 105 colony-forming units of M. bovis, and the 0.05 mL dose for infants contains 1–4 × 105 colony-forming units. Other components and residuals include sodium glutamate, magnesium sulphate heptahydrate, dipotassium phosphate, citric acid, L‑asparagine monohydrate, ferric ammonium citrate and glycerol.
23.4.2. Efficacy and effectiveness
23.4.2. Efficacy and effectiveness
The exact immune response elicited by BCG vaccination and the mechanism of action in the host are still not well understood. There is no reliable established laboratory correlate for immunity to M. tuberculosis,[12] though this remains an active area of study.[13]
BCG protection is partial and varies according to the age at which vaccination is administered and the disease phenotype in question. A meta-analysis of randomised controlled trials showed neonatal BCG had 59 percent efficacy against pulmonary TB (95% CI: 0.42–0.71) and 90 percent efficacy against meningeal TB (95% CI: 0.23–0.99). [14] Studies conducted since the advent of interferon gamma release assays suggest BCG may also be effective against M. tuberculosis infection. A meta-analysis of 26 cohort studies has estimated the overall effectiveness of BCG against all tuberculosis at 18 percent (aOR 0.82, 95% CI 0.74-0.91).[15] When stratified by age, BCG vaccination at birth only significantly protected children under 5 years (aOR 0.63, 0.49-0.81). The study concluded that BCG vaccination at birth is effective at preventing TB in young children but is ineffective in adolescents and adults.[15] Thus, the principal role of BCG in New Zealand is to protect young children who are at greatest risk of disease, particularly miliary and meningeal disease.[11] BCG is less effective in adults and older children, particularly if they already have latent infection.
As BCG has been propagated in vitro for over 40 years, there are now several strains being manufactured.[16] Immunological responses vary considerably across vaccine strains, but the data to date cannot differentiate which strains, if any, are overall more effective.[17, 18]
In low-income countries, a birth dose of BCG significantly reduces overall infant mortality.[19, 20]
BCG has had little effect in reducing the population rate and transmission of TB,[21] so there are no herd immunity effects. Duration of protection is unknown, possibly 10 to 15 years, but it may be much longer in some populations.[11]
There have been a number of different approaches to using BCG in the control of TB in middle- and high-income countries.[22] For example, the US has not had a BCG programme, whereas New Zealand (see Appendix 1) and the UK had programmes until 1990 and 2005, respectively. The WHO recommends that countries with low rates of active TB, such as New Zealand, target BCG vaccination at children who are at significantly increased risk of TB exposure through household contact.[7] New Zealand (see section 23.5) and the UK now only offer BCG vaccine to high-risk individuals. A study from the Netherlands suggests that around 9,000 children from countries with rates greater than 50 per 100,000 population would have to be given BCG to prevent a severe case.[23]
The current recommendation to use neonatal BCG vaccination in populations with high rates of active TB is part of a control and treatment programme for TB in New Zealand, which includes active contact tracing and treatment of latent TB infection.
There are large international efforts working to improve BCG vaccines and develop new, more effective vaccines.[24]
23.4.3. Transport, storage and handling
23.4.3. Transport, storage and handling
Transport according to the National Standards for Vaccine Storage and Transportation for Immunisation Providers 2017 (2nd edition).
Store in the dark at +2°C to +8°C. Do not freeze.
There are variances in strain potency between brands of BCG vaccine so vaccinators should always follow the instructions in the vaccine data sheet (available on the Medsafe website (external link)).
BCG vaccine requires reconstitution before administration. It is presented as freeze-dried vaccine in a multi-dose vial with diluent in a separate vial. The diluent must be added to the freeze-dried vaccine vial and mixed gently (do not shake vigorously). Protect the vial from light. Leave the reconstituted vaccine to stand for one minute until it forms an opalescent liquid. Reconstituted vaccine should be stored at 4°C, protected from sunlight and used within four hours.
23.4.4. Dosage and administration
23.4.4. Dosage and administration
Only authorised vaccinators with BCG endorsement can administer BCG vaccine (see A3.6 (external link)).
Administer a dose of:
- 0.05 mL to infants aged under 12 months
- 0.1 mL to children aged 12 months or older.
The vaccine is administered by intradermal injection over the point of insertion of the left deltoid muscle (see sections 2.2.3 and 2.2.4 (external link)).
No follow-up tuberculin skin testing is required.
Repeat BCG vaccination is not recommended.
BCG immunisation given in other countries
BCG is one of the vaccines that are part of the WHO Expanded Programme on Immunization. It is given at birth in most low-income countries.
The following Pacific Island countries[25] recommend BCG vaccination at birth: Cook Islands, Fiji, Kiribati, Nauru, Niue, Papua New Guinea, Samoa, Solomon Islands, Tonga, Tuvalu and Vanuatu.
Usually BCG vaccine is administered in the left deltoid area, but other sites of administration have also (although uncommonly) been used, such as the right deltoid. Revaccination with BCG is not recommended by the WHO.[7 (external link)]
Co-administration with other vaccines
BCG can be given simultaneously with any other vaccine. However, it must be administered into a separate site in a separate syringe. Because of the risk of local lymphadenitis, no further vaccinations should be given into the arm used for BCG for at least three months. If not given concurrently, BCG should be given at least four weeks after MMR or VV. Note that no time interval is required between administration of BCG and rotavirus vaccines.
HBIG (given at birth to babies of mothers with chronic HBV infection) or human normal immunoglobulin is thought not to reduce the effectiveness of BCG immunisation, which principally acts through cell‑mediated immunity.
23.5. Recommended immunisation schedule
23.5.1. Tuberculin skin testing (Mantoux) or interferon-gamma release assay before BCG vaccination
23.5.1. Tuberculin skin testing (Mantoux) or interferon-gamma release assay before BCG vaccination
Tuberculin skin testing is not needed if BCG is given before age 6 months unless a history of contact with a known or possible case of TB is obtained. Although the tuberculin skin test is usually positive in the year following BCG vaccination, at least 50 percent of children will be negative beyond that time, so tuberculin skin testing still has utility for diagnosing TB infection.
Children eligible for BCG vaccine who have missed vaccination at birth may be vaccinated at any time up to age 5 years (see section 23.5.3 (external link)). If the child is aged 6 months to <2 years, they should have a pre-vaccination tuberculin skin test or an interferon-gamma release assay (IGRA; Quantiferon) if aged 2 to <5 years to detect whether they have already been infected: BCG vaccination only to be given if the child is uninfected.
Live virus vaccines, including MMR and varicella, can reduce the response to tuberculin skin testing and IGRA blood test results.[26, 27] Either conduct a TB test on the same day as a MMR or VV vaccination or postpone for at least four weeks after vaccination.[26, 27] TB testing can be conducted at any time after vaccination with non-live vaccines.
23.5.2. BCG eligibility criteria
23.5.2. BCG eligibility criteria
TB is more common in migrants or families of migrants from high-incidence countries. However, anyone who is pregnant should have a discussion with their lead maternity carer about the risk of TB for their baby.
Neonatal BCG is recommended and funded for infants at increased risk of TB, as defined in Table 23.1 (external link).
Table 23.1: Neonatal BCG eligibility criteria
|
Neonatal BCG is recommended and funded for infants at increased risk of TB, defined as those who:
|
|
* For TB incidence rates in specific countries see the Tuberculosis Profile website (external link). |
The WHO Global Tuberculosis 2021 report showed that the highest burden for TB was seen in 30 countries. For more detail see the Global Tuberculosis Report 2021 (external link).
As a general indication, the following global areas have TB rates ≥40 per 100,000:
- most of Africa
- much of South America
- Russia and the former Soviet states
- the Indian subcontinent
- Eastern Mediterranean
- China (including Hong Kong) and Taiwan
- South-East Asia
- some parts of the Pacific (Kiribati and Papua New Guinea have consistently high rates).
For TB incidence rates in specific countries see the Tuberculosis Profile website (external link).
Neonates at risk should be identified antenatally by lead maternity care providers and antenatal referral made to the neonatal BCG service. Health care providers can also identify and refer neonates at risk. Immunisation is desirable before infants leave hospital. If this does not happen, immunisation should be arranged through the local medical officer of health.
Infants born before 34 weeks’ gestation should have their BCG vaccination delayed until 34 weeks’ post-conceptual age.[28] Babies born after this or with low birthweight appear to produce an adequate response, based on tuberculin skin test responses.[29, 30]
If the baby has not been vaccinated before leaving hospital, and if there is a history of current TB in a relative who has had contact with the baby, do not vaccinate immediately. Withhold vaccination, conduct tuberculin skin testing, seek paediatric advice and vaccinate only after the possibility of infection in the baby has been excluded. Vaccination may not protect the baby who is incubating disease and may prevent the tuberculin test from assisting with the diagnosis of disease.
A parent’s/guardian’s request should not be accepted as an indication for immunisation. Parents/guardians seeking vaccination of children who do not meet the above criteria should be referred to the local medical officer of health to discuss the risks and benefits of immunisation before a final decision is made.
BCG vaccine information for parents
Information about the BCG vaccine is available in English and other languages from the HealthEd website (external link). This includes information for parents on why the vaccine is recommended, what to expect and how to care for the vaccination site.
23.5.3. Children aged under 5 years at high TB risk
23.5.3. Children aged under 5 years at high TB risk
Repeat BCG vaccination is not recommended. Vaccination for overseas travel is not available in New Zealand.
Funded BCG may be offered to children aged under 5 years if they are tuberculin skin test- or interferon gamma-release assay negative and are at increased risk of TB because they:
- will be living in a house or family/whānau with a person with either current TB or a history of TB or
- have one or both parents, household members or carers who within the last five years lived for a period of six months or longer in countries with a TB rate ≥40 per 100,000* or
- during their first five years will be living for three months or longer in a country with a TB rate ≥40 per 100,000*.
* For TB incidence rates in specific countries see t (external link)he Tuberculosis profile (external link).
23.5.4. Pregnancy and breastfeeding
23.5.4. Pregnancy and breastfeeding
BCG vaccine is not routinely recommended for when someone is pregnant or breastfeeding.
23.6. Contraindications and precautions
See also section 2.1.3 (external link)for pre-vaccination screening guidelines and section 2.1.4 for general contraindications for all vaccines.
23.6.1. Contraindications
23.6.1. Contraindications
BCG vaccine should not be given to individuals:
- known to be hypersensitive to any component of the vaccine
- receiving corticosteroids or other immunosuppressive treatment, including radiotherapy (see section 4.3)
- suffering from malignant conditions such as lymphoma, leukaemia, Hodgkin’s disease or other tumours of the reticulo-endothelial system
- in whom immunocompromise is known or suspected, such as individuals with hypogammaglobulinaemia – primary immune deficiencies in children are often not detected until after the first few weeks of life (ie, after BCG vaccine is given), so a family history of immune deficiency should be sought and, if present, discussed with a paediatrician before vaccination
- known to be infected with HIV, including neonates where the mother’s HIV status is unknown – maternal HIV infection should be excluded prior to neonatal vaccination; testing should have been offered as part of the National Antenatal HIV Screening Programme, and infants born to HIV-infected mothers should be under the care of a paediatrician
- with generalised infected skin conditions.
Infants born to those who received immunomodulatory biologic agents during pregnancy must not be vaccinated with a BCG vaccine until they are identified as being immunocompetent. See section 4.2.5 (external link) and Table 4.2 for a list of the highly immunosuppressive medications with long half-lives that require a prolonged delay before vaccination (for up to one year in those being treated). These include monoclonal antibody (mab) agents that readily cross the placenta. Each case should be assessed with specialist advice.
23.6.2. Precautions
23.6.2. Precautions
- BCG vaccine should be avoided in those who are pregnant (this is a counsel of caution, as no harmful effects to the fetus have been observed following accidental immunisation of the mother during pregnancy).
- In the case of eczema, an immunisation site should be chosen that is free of skin lesions.
- Infants born before 34 weeks’ gestation should have their BCG vaccination delayed until 34 weeks’ post-conceptual age.[28]
- Avoid or defer immunisation in a child born with a condition that may require immunosuppressive therapy in future.
- Before BCG vaccination is scheduled for neonates (up to 4 weeks of age), a normal metabolic/immune deficiency (Guthrie test) result needs to have been confirmed, specifically for severe combined immunodeficiency (SCID).
23.7. Potential responses and AEFIs
23.7.1. Potential responses
23.7.1. Potential responses
Following the BCG injection, a white weal should appear. This should subside in approximately 30 minutes. The site requires no swabbing or dressing.
A local reaction develops in 90–95 percent of people vaccinated with BCG, which may include shallow ulceration, followed by healing and scar formation within three months. To ensure appropriate healing, encourage parents/caregivers to keep the injection site clean and dry, to allow sore to scab and to avoid ointments and scratching. A minor degree of adenitis developing in the weeks following immunisation should be regarded as normal, not a complication. It may take months to resolve. Suppurative adenitis may also take months to resolve; usually no treatment is required.
23.7.2. AEFIs
23.7.2. AEFIs
AEFIs with BCG vary with age and vaccine strain and are summarised in Table 23.2.
Table 23.2: Age-specific estimated risks for complications after administration of BCG vaccine
|
Complication |
Incidence per 1 million vaccinations |
||
|---|---|---|---|
|
Age <1 year |
Age 1–20 years |
||
|
Local subcutaneous abscess; regional lymphadenopathy |
387 |
25 |
|
|
Musculoskeletal lesions |
0.39–0.89 |
0.06 |
|
|
Multiple lymphadenitis; non-fatal disseminated lesions |
0.31–0.39 |
0.36 |
|
|
Fatal disseminated lesions |
0.19–1.56 |
0.06–0.72 |
|
|
Reprinted with permission of the International Union Against Tuberculosis and Lung Disease. Copyright © The Union. Lotte A, Wasz-Hockert O, Poisson N, et al. 1988. Second IUATLD study on complications induced by intradermal BCG-vaccination. Bulletin of the International Union against Tuberculosis and Lung Disease 63: 47–59. |
|||
The risk of BCG adverse reactions depends on many factors, including strain type, route of administration and the underlying immune state of the patient. Severe injection-site reactions, large ulcers and abscesses can occur in individuals who are tuberculin positive. Special care is needed both in interpreting initial tuberculin skin results and in delivering the BCG vaccine.
Rarely, osteitis and osteomyelitis, lupoid and other types of skin disorders, and neurological disorders have been reported following BCG vaccination. Although rare, disseminated BCG disease is the most severe BCG vaccine complication occurring in immunocompromised people, such as children with primary immune deficiency. This needs rapid and aggressive treatment and has a high mortality.
Keloid scars at the injection-site, although not uncommon, are largely avoidable. Some sites are more prone to keloid formation than others and vaccinators should adhere to the site recommended (mid-upper arm). Most experience has been with the upper arm site, and it is known that the risk of keloid formation increases greatly if the injection is given higher than the insertion of the deltoid muscle into the humerus.
Every effort should be made to recover and identify the causative organism from any lesions that constitute a serious complication.
Most local and regional adenopathy resulting from BCG vaccination will resolve spontaneously, and there is rarely a need for medical or surgical intervention. Treatment recommendations for local abscess formation and suppurative lymphadenitis remain controversial.[31] If suppurative adenitis reactions persist for longer than three months, seek specialist opinion. However, anyone presenting with more widespread or distant disease needs referral to a specialist.
Abscesses and more serious complications should be reported to CARM (see ‘AEFI reporting process – notifying CARM’ in section 1.6.3), and also reported to the local medical officer of health in the interests of quality control of the BCG vaccination technique.
23.8. Public health measures
It is a legal requirement that all cases of active TB be notified to the local medical officer of health. While there is no legal requirement to notify cases of latent TB infection that are being treated, for surveillance purposes and with the patient’s consent they should be reported to the local medical officer of health.
For more information refer to the 'Tuberculosis' chapter of the Communicable Disease Control Manual and see the 'Tuberculosis' page for the health sector guidance available on the Health New Zealand | Te Whatu ora website.
Under the Health (Protection) Amendment Act 2016, the medical officer of health is given wide powers to investigate and control all TB cases and their contacts, while districts are required to make provision for the treatment and supervision of patients and their contacts.
The primary purpose of neonatal BCG vaccination is to protect child case contacts from TB disease and its most devastating consequences. Screening of certain risk groups and case contact management are other elements of TB control in New Zealand. These programmes do not obviate the need for BCG vaccination, as screening coverage is partial and contact tracing may not occur in time to prevent illness in child contacts. The local medical officer of health can advise on local TB control policies, including issues in BCG immunisation.
Both TB infection and BCG immunisation lead to the development of a cellular immune response, which can be detected by measuring dermal induration after the injection of tuberculin-purified protein derivative (eg, via the tuberculin skin test). A positive response to a tuberculin skin test may be an indication of current infection, previous natural infection or prior BCG immunisation. However, the false positive effect after vaccination will wane, rapidly in all individuals who receive the vaccine in the neonatal period and more slowly in those who are vaccinated at an older age such as during the primary-school years.[32]
In vitro tests have been developed to measure the release of interferon-gamma from host lymphocytes in response to well-defined antigens. The antigens used are not present in BCG strains of M. bovis or most non‑tuberculous mycobacteria. Interferon gamma release assay has the advantage of greater specificity and convenience, but it is more expensive.[33]
23.9. Variations from the vaccine data sheet
The data sheet states that BCG vaccine should not be given to infants born to HIV‑positive mothers. Health NZ | Te Whatu Ora recommends that BCG may be given to HIV‑negative infants born to HIV-positive mothers – providing that the infant is confirmed to be HIV negative by appropriately-timed PCR tests before the vaccine is given.[34, 35] Seek specialist advice.
References
References
References
1. Getahun H, Matteelli A ,Chaisson R. Latent Mycobacterium tuberculosis infection. New England Journal of Medicine, 2015. 372(22): p. 2127–35.
2. Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. International Journal of Tuberculosis and Lung Disease, 2004. 8(4): p. 392-402.
3. World Health Organization. 2018 Latent tuberculosis infection: updated and consolidated guidelines for programmatic management.: Geneva. URL: https://www.who.int/publications/i/item/9789241550239 (external link). (accessed 20 August 2024)
4. World Health Organization. 2018 Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. World Health Organization,| CC BY-NC-SA 3.0 IGO. URL: https://www.who.int/publications/i/item/9789241550239 (external link). (accessed 24 September 2024)
5. Schaaf H ,Zumla Ae, Tuberculosis: A Comprehensive Clinical Reference. 2009, London, UK: WB Saunders Elsevier.
6. Global Tuberculosis Programme (GTB). 2023 Global tuberculosis report 2023. World Health Organization. URL: https://www.who.int/publications/i/item/9789240083851 (external link). (accessed 24 September 2024)
7. World Health Organization. BCG vaccines: WHO position paper – February 2018. Weekly Epidemiological Record, 2018. 93(8): p. 73-96.
8. Pareek M, Watson JP, Ormerod LP, et al. Screening of immigrants in the UK for imported latent tuberculosis: a multicentre cohort study and cost-effectiveness analysis. Lancet Infectious Diseases, 2011. 11(6): p. 435-44.
9. Institute of Environmental Science and Research Ltd. 2016. Notifiable Diseases in New Zealand: Annual Report 2015 (ed.), Porirua, New Zealand: The Institute of Science and Environmental Research Ltd. URL: https://surv.esr.cri.nz/PDF_surveillance/AnnualRpt/AnnualSurv/2015/2015AnnualReportFinal.pdf (external link) (accessed 3 July 2020)
10. Institute of Environmental Science and Research. 2023 Tuberculosis in New Zealand: Annual Report 2020.: Porirua, New Zealand. (accessed
11. Hanekom W, Hawn T ,Ginsberg A. 2018. Tuberculosis Vaccines, in Plotkin's Vaccines (7th Edition), Plotkin S, Orenstein W, Offit P, and Edwards K (eds). Elsevier: Philadelphia, US.
12. Nunes-Alves C, Booty MG, Carpenter SM, et al. In search of a new paradigm for protective immunity to TB. Nature Reviews: Microbiology, 2014. 12(4): p. 289-99.
13. Tanner R, O'Shea MK, Fletcher HA,McShane H. In vitro mycobacterial growth inhibition assays: A tool for the assessment of protective immunity and evaluation of tuberculosis vaccine efficacy. Vaccine, 2016. 34(39): p. 4656-4665.
14. Mangtani P, Abubakar I, Ariti C, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clinical Infectious Diseases, 2014. 58(4): p. 470-80.
15. Martinez L, Cords O, Liu Q, et al. Infant BCG vaccination and risk of pulmonary and extrapulmonary tuberculosis throughout the life course: a systematic review and individual participant data meta-analysis. Lancet Glob Health, 2022. 10(9): p. e1307-e1316.
16. Copin R, Coscolla M, Efstathiadis E, et al. Impact of in vitro evolution on antigenic diversity of Mycobacterium bovis bacillus Calmette-Guerin (BCG). Vaccine, 2014. 32(45): p. 5998-6004.
17. Anderson EJ, Webb EL, Mawa PA, et al. The influence of BCG vaccine strain on mycobacteria-specific and non-specific immune responses in a prospective cohort of infants in Uganda. Vaccine, 2012. 30(12): p. 2083-9.
18. Ritz N, Hanekom WA, Robins-Browne R, et al. Influence of BCG vaccine strain on the immune response and protection against tuberculosis. FEMS Microbiology Reviews, 2008. 32(5): p. 821-41.
19. Thysen SM, Benn CS, Gomes VF, et al. Neonatal BCG vaccination and child survival in TB-exposed and TB-unexposed children: a prospective cohort study. BMJ Open, 2020. 10(2): p. e035595.
20. Biering-Sørensen S, Jensen KJ, Monterio I, et al. Rapid protective effects of early BCG on neonatal mortality among low birth weight boys: observations from randomized trials. Journal of Infectious Diseases, 2018. 217(5): p. 759-766.
21. World Health Organization. BCG Vaccine. URL: https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/norms-and-standards/vaccines-quality/bcg (external link). (accessed 24 September 2024)
22. Zwerling A, Behr MA, Verma A, et al. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Medicine, 2011. 8(3): p. e1001012.
23. Altes HK, Dijkstra F, Lugner A, et al. Targeted BCG vaccination against severe tuberculosis in low-prevalence settings: epidemiologic and economic assessment. Epidemiology, 2009. 20(4): p. 562-8.
24. Ottenhoff TH ,Kaufmann SH. Vaccines against tuberculosis: where are we and where do we need to go? PLoS Pathogens, 2012. 8(5): p. e1002607.
25. World Health Organization, WHO Vaccine-preventable Diseases: Monitoring system: 2016 global summary. 2016: Online.
26. US Centers for Disease Control and Prevention (CDC). 2024 Clinical testing guidance for tuberculosis: interferon gamma release assay. 2024 [updated 9 May 2024 ]; URL: https://www.cdc.gov/tb/hcp/testing-diagnosis/interferon-gamma-release-assay.html (external link). (accessed 15 February 2025)
27. American Academy of Pediatrics. 2018. Measles. in Red Book: 2018 Report of the Committee on Infectious Diseases, Kimberlin D, Brady M, Jackson M, and Long S (eds). Elk Grove Village, IL. p. 537-550. URL: https://redbook.solutions.aap.org/redbook.aspx (external link). (accessed 3 July 2020)
28. Sedaghatian MR, Hashem F ,Moshaddeque Hossain M. Bacille Calmette-Guérin vaccination in pre-term infants. International Journal of Tuberculosis and Lung Disease, 1998. 2(8): p. 679-82.
29. Badurdeen S, Marshall A, Daish H, et al. Safety and Immunogenicity of Early Bacillus Calmette-Guerin Vaccination in Infants Who Are Preterm and/or Have Low Birth Weights: A Systematic Review and Meta-analysis. JAMA Pediatr, 2019. 173(1): p. 75-85.
30. Saroha M, Faridi MM, Batra P, et al. Immunogenicity and safety of early vs delayed BCG vaccination in moderately preterm (31-33 weeks) infants. Human Vaccines & Immunotherapeutics, 2015. 11(12): p. 2864-71.
31. Caglayan S, Yegin O, Kayran K, et al. Is medical therapy effective for regional lymphadenitis following BCG vaccination? American Journal of Diseases of Children, 1987. 141(11): p. 1213-4.
32. Farhat M, Greenaway C, Pai M,Menzies D. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? International Journal of Tuberculosis and Lung Disease, 2006. 10(11): p. 1192-204.
33. Centers for Disease Control and Prevention. Updated guidelines for using interferon gamma release assays to detect mycobacterium tuberculosis infection – United States, 2010. Morbidity and Mortality Weekly Report: Recommendations and Reports, 2010. 59(RR05): p. 1–25.
34. UK Health Security Agency. 2018. Tuberculosis. in The Green Book. URL: https://www.gov.uk/government/publications/tuberculosis-the-green-book-chapter-32 (external link). (accessed 24 September 2024)
35. Australian Technical Advisory Group on Immunisation. 2018. Australian Immunisation Handbook (ed.), Canberra: Australian Government Department of Health. URL: https://immunisationhandbook.health.gov.au/ (external link) (accessed October 2019)