On this page
Key information
|
Mode of transmission |
Airborne droplets or by direct contact with saliva or urine from an infected person. |
|---|---|
|
Incubation period |
About 16 to 18 days, ranging from 12 to 25 days. |
|
Period of communicability |
For contact tracing purposes, the recommended period of communicability is from 2 days before to 5 days after the onset of parotitis. The virus is also transmitted by asymptomatic infections. |
|
Incidence and burden of disease |
Outbreaks are continuing to occur in New Zealand. |
|
Funded vaccine |
MMR (Priorix) is a live attenuated vaccine. |
|
Dose, presentation, route |
0.5 mL per dose after reconstitution. Pre-filled syringe and glass vial. The vaccine must be reconstituted prior to injection. Intramuscular or subcutaneous injection. |
|
Funded vaccine indications and schedule |
Children at ages 12 months and 15 months. Adults who are susceptible to one or more of measles, mumps and rubella. For (re)vaccination following immunosuppression (if the individual is immunocompetent enough to safely receive the vaccine). |
|
Recommended |
All adults born since January 1969 should be up to date with two doses of MMR or have evidence of immunity to all three vaccine components. It is particularly important for health care workers, individuals who work with children, armed forces personnel, staff of correctional facilities, long-term care facilities and immigration/refugee centres and laboratory staff. All vaccine-eligible travellers, particularly to high-risk countries |
|
Vaccine effectiveness |
After one dose, MMR is 64–66 percent effective against laboratory-confirmed mumps and 83–88 percent after two vaccine doses. |
|
Duration of protection |
At least 10 years; protection is best achieved via herd immunity from high immunisation coverage. |
|
Contraindication and precautions |
MMR is contraindicated for anaphylaxis to neomycin, immunocompromise and in pregnancy. See section 12.6 for cautions around receipt of blood products and other live vaccines, and other precautions. MMR may temporarily suppress tuberculin skin test reactivity. |
|
Potential responses to vaccine |
MMR is generally well tolerated. There can be mild salivary gland swelling after 10–14 days. If there is fever and rash 6–12 days after vaccination this is due to measles and rubella components. Alert parents of possible febrile seizure risk, particularly for those with a history of seizure. |
|
Public health measures |
Notify the local medical officer of health immediately on suspicion of wild-type mumps. (see section 15.8) |
15.1. Virology
Mumps is a paramyxovirus, genus Rubulavirus, with a single-stranded RNA genome. It is rapidly inactivated by heat, formalin, ether, chloroform and light.
15.2. Clinical features
Mumps is transmitted by airborne droplets or direct contact with infected respiratory tract secretions or urine. Humans are the only known host of the virus.
People with mumps are most infectious from two days before to five days after the onset of parotitis;[1] therefore, this is the recommended period of communicability for contact tracing purposes.[1] However, mumps virus has been isolated in saliva from seven days before to nine days after the onset of parotitis. Asymptomatic cases also can be infectious.[1]
Classic mumps, an acute viral illness, is characterised by fever, headache, and swelling and tenderness of one or more parotid (salivary) glands. Mumps starts as an upper respiratory tract infection that disseminates via plasma viremia to glandular tissue, kidneys and central nervous system. Some patients may experience involvement of other organs (eg, orchitis or meningitis) without salivary gland involvement. At least 30 percent of mumps infections in children are asymptomatic.[2]
The complications of symptomatic mumps include clinically evident aseptic meningitis in 5–10 percent (almost always without sequelae), orchitis (usually unilateral) in up to one-third of post-pubertal males, and oophoritis and mastitis in 5–30 percent of post-pubertal females. Sterility is exceedingly rare in males and unconfirmed in females.[2] Profound sudden onset unilateral nerve deafness occurs in 1 in 15,000–20,000 cases. Pancreatitis, neuritis, arthritis, myelitis, nephritis, thyroiditis and pericarditis may also occur.[3]
Encephalitis has been reported to occur at a frequency of between 1 in 400 and 1 in 6,000, the latter being a more realistic estimate. The case fatality rate for mumps encephalitis is 1.4 percent, while the overall mumps case fatality rate is reported as 1.8 per 10,000 cases. Mumps in the first trimester of pregnancy may increase the rate of spontaneous abortion, but there is no evidence that it causes fetal abnormalities.
15.3. Epidemiology
15.3.1. Global burden of disease
15.3.1. Global burden of disease
Prior to the introduction of immunisation, approximately 85 percent of adults had evidence of past mumps infection. Most infections in those aged under 2 years are subclinical, while those affected in adulthood are more likely to experience severe disease. The peak incidence was in late winter and spring.
More recently, there have been numerous reports of increasing numbers of mumps cases in the US, the UK and elsewhere, thought to be due to a waning of vaccine-induced immunity.[4] Many cases are reported in 18–30-year olds.[5] Outbreaks appear to occur mainly in those in crowded situations, such as university students and other close-knit communities, and have been associated with international travel.[6]
15.3.2. New Zealand epidemiology
15.3.2. New Zealand epidemiology
Mumps vaccine (as MMR) was introduced to the Schedule in 1990 for children aged 12 to 15 months, with a second dose introduced in 1992 for children aged 11 years. A two-dose schedule at ages 15 months and 4 years was introduced in 2001 (see Appendix 1 for more information). Prior to 2017, the last mumps epidemic had occurred in 1994. The current two-dose schedule was introduced in 2020 at age 12 months and 15 months.
People aged 12–29 years are at the greatest risk of catching mumps, as they are the group least likely to have been fully immunised as children. Those born in Fiji, Tonga, Kiribati, Nauru, Papua New Guinea, Solomon Islands, Tuvalu and Vanuatu as well as many mainland nations in Asia may not have been offered mumps immunisation as children.
From 1 January 2017 to 31 December 2019, 1,773 cases of mumps were notified (ESR, 8 June 2020). During 2019, the notification rate was 5.4 per 100,000 (264 cases) compared with 9.0 per 100,000 (435 cases) in 2018. In 2019, the highest notification rates were seen in young adults aged 15 to 29 years. Cases were predominantly in the Auckland region, with the highest notification rates in the Middle Eastern/Latin American/African (MELAA) ethnic group (19.9 per 100,000) and Pacific people (15.2 cases per 100,000; ESR, 8 June 2020).
Figure 15.1: Notified cases of mumps, 1997–2019
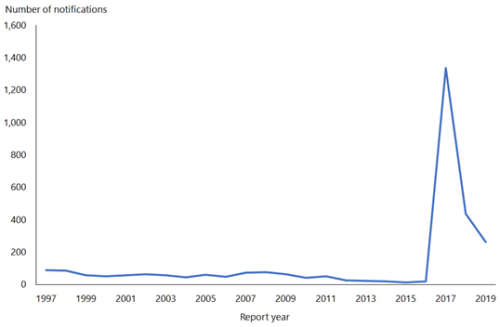
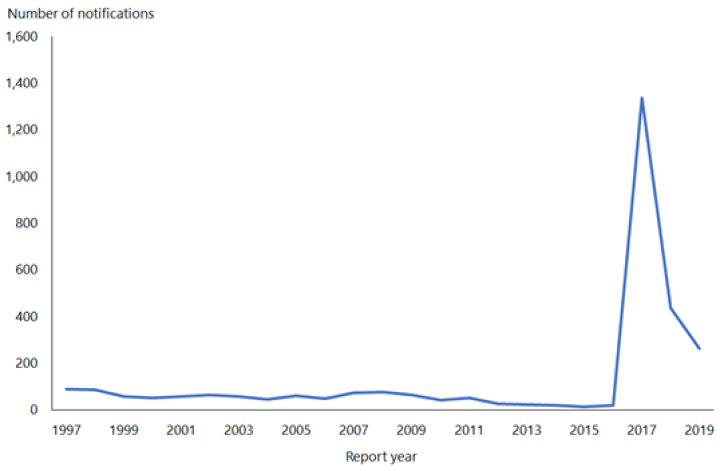
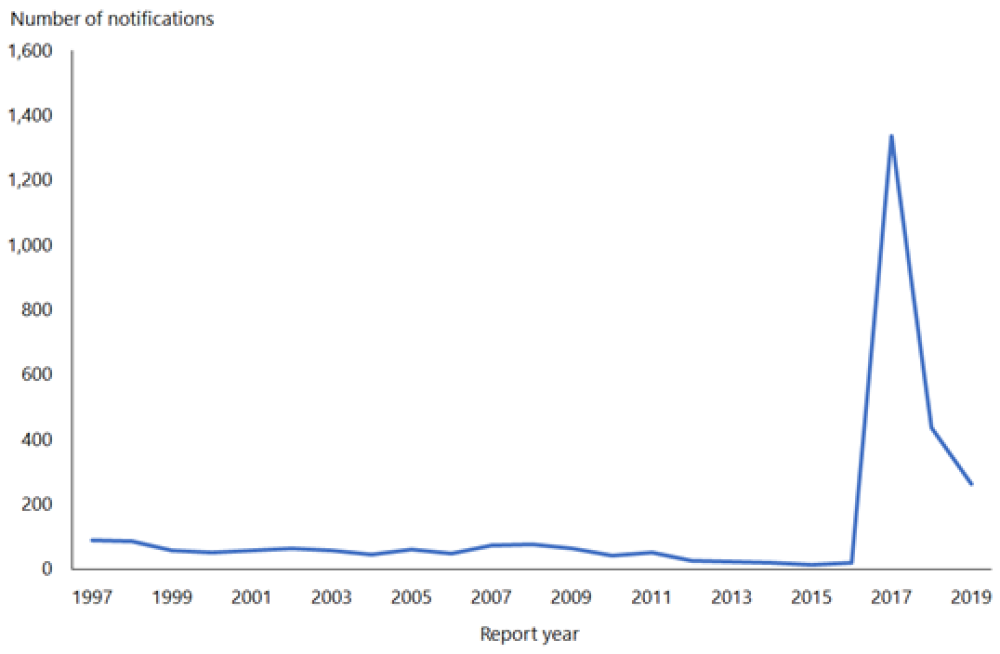
Source: ESR
Outbreaks continue to occur throughout New Zealand. For further details refer to the PHF Science (formerly ESR) surveillance reports for notifiable diseases (external link).
15.4. Vaccines
15.4.1. Available vaccines
15.4.1. Available vaccines
Mumps vaccine is one of the components of the live attenuated MMR, considered in section 12.4 (and MMRV mentioned in section 24.4). There are no single antigen mumps vaccines available.
Funded vaccine
MMR vaccine funded as part of the Schedule is Priorix (GSK), which contains attenuated Schwarz strain measles, RA 27/3 rubella and Jeryl Lynn mumps. See section 12.4.1 for more information
Other vaccines
M-M-R II (MSD) was the funded vaccine prior to the 1 July 2017 Schedule change (see section 12.4.1).
15.4.2. Efficacy and effectiveness
15.4.2. Efficacy and effectiveness
A 2012 Cochrane review of the safety and effectiveness of MMR estimated that a single dose of MMR was 69–81 percent effective in preventing clinical mumps. Effectiveness of MMR in preventing laboratory-confirmed mumps cases in children and adolescents was estimated to be between 64 and 66 percent for one dose and between 83 and 88 percent for two vaccine doses.[7]
A two-dose vaccination schedule and high immunisation coverage has been very successful in controlling disease. However, outbreaks can still occur in highly immunised populations, because two doses of vaccine are not 100 percent effective. Community coverage needs to be 85–90 percent by 2 years of age to prevent outbreaks from asymptomatic cases.[2] Declining vaccine-induced mumps immunity may also contribute to outbreaks.[4] Data from Finland shows that 20 years after the second MMR dose, immunity to mumps declined with just under 75 percent being seropositive.[8] The antibody avidity also decreased over time, by 24 percent for mumps.[9]
Two doses of MMR are required to ensure a high rate of seroprotection against mumps and induce antibody responses that persist for at least 10 years post-vaccination.[10, 11] However, waning in immunity has been observed in young adults and a third dose of MMR has been used safely and effectively during mumps outbreaks in highly immunised populations.[12, 13] Although the mumps vaccine is not quite as effective as measles and rubella vaccines, cases that have been vaccinated are significantly less likely to experience complications from disease such as orchitis, meningitis and hospitalisation.[14]
15.4.3. Transport, storage and handling
15.4.3. Transport, storage and handling
Transport according to the National Standards for Vaccine Storage and Transportation for Immunisation Providers 2017 (2nd edition).
Store at +2°C to +8°C. Do not freeze.
MMR must be reconstituted only with the diluents supplied by the manufacturer. Use MMR as soon as possible after reconstitution. If storage is necessary, reconstituted vaccine can be stored at +2°C to +8°C for up to eight hours.
15.4.4. Dosage and administration
15.4.4. Dosage and administration
The dose of MMR is all of the reconstituted vaccine (approximately 0.5 mL) administered by intramuscular injection, or subcutaneous injection if indicated (see section 2.2.3).
Co-administration with other vaccines
MMR can be given concurrently with other vaccines, by using separate syringes and giving the injections at different sites. If not given concurrently, live vaccines should be given at least four weeks apart. (See also section 2.2.7 for information about multiple injections at the same visit.)
Interchangeability
The two brands of MMR available in New Zealand (Priorix and M-M-R II) may be used interchangeably for completion of a course.[15]
15.5. Recommended immunisation schedule
Table 15.1: Recommended MMR vaccination schedule
Table 15.1: Recommended MMR vaccination schedule
|
|
Schedule |
|---|---|
|
Usual childhood schedulea |
2 doses: at ages 12 months and 15 months |
|
Catch-upb for children, adolescents and adults |
2 doses: at least 4 weeks apart |
|
a. If MMR is given to children aged under 12 months for outbreak control, 2 further MMR doses are still required at ages 12 months (at least 4 weeks after previous dose) and 15 months. b. MMR is funded for those who are susceptible to one or more of the three diseases. |
|
15.5.1. Usual childhood schedule
15.5.1. Usual childhood schedule
Two doses of mumps-containing vaccine as MMR are recommended at age 12 months and 15 months (Table 15.1).
The second dose can be given as soon as four weeks after the first dose.
Children who receive MMR when aged under 11 months (MMR0) during an outbreak require two further doses administered after age 12 months. No opportunity should be missed to achieve immunity.
15.5.2. Catch-up
15.5.2. Catch-up
MMR is recommended and funded for children, adolescents and adults (especially those born since 1 January 1969) who are known to be susceptible to one or more of the three diseases (two doses, four weeks apart). See sections 12.5.2 and 21.5.2.
15.5.3. Immunocompromise
15.5.3. Immunocompromise
Contacts of immunocompromised individuals
In general, MMR is contraindicated in immunocompromised individuals (see section 4.2.5). These individuals can be partially protected from exposure to infection by ensuring that all contacts are fully immunised (funded), including hospital staff and family members. There is no risk of transmission of MMR vaccine viruses from a vaccine recipient to the immunocompromised individual (see section 12.7.2).
See also in section 4.3.1.
(Re)vaccination following immunosuppression
MMR is funded for (re)vaccination following immunosuppression. However, it is important to be sure that the individual is immunocompetent enough to safely receive the vaccine.
HIV infection
Discuss vaccination of individuals with HIV infection with their specialist (see ‘HIV infection’ in section 4.3.12).
MMR is recommended for all HIV-positive children, whether they are symptomatic or asymptomatic, if their CD4+ lymphocyte percentage is 15 percent or greater. Asymptomatic children who are not severely immunocompromised are recommended to receive MMR from age 12 months to provide early protection against the three diseases. Susceptible HIV-positive children and adults aged 14 years and older may receive MMR if their CD4+ lymphocyte count is at least 200 cells/mm3. Administration of MMR with CD4+ counts below these recommended levels has been associated with vaccine-related pneumonitis (from the measles component).[16]
15.5.4. Pregnancy and breastfeeding
15.5.4. Pregnancy and breastfeeding
MMR is contraindicated during pregnancy. Pregnancy should be avoided for four weeks after MMR vaccination.[16, 17] (see section 15.6.1).
After delivery
If MMR and Rhesus anti-D IG are required after delivery, both the vaccine and anti-D IG may be given at the same time, in separate sites with separate syringes. The vaccine may be given at any time after the delivery. Anti-D IG does not interfere with the antibody response to the vaccine, but whole blood transfusion does inhibit the response in up to 50 percent of vaccines recipients (see section A6.4.1). MMR can safely be given to breastfeeding women.
15.6. Contraindications and precautions
See also section 2.1.3 for pre-vaccination screening guidelines and section 2.1.4 for general vaccine contraindications.
15.6.1. Contraindications
15.6.1. Contraindications
See section 12.6.1 for specific MMR contraindications.
Anaphylaxis to a previous dose of MMR or any of the vaccine components (including neomycin) is a contraindication to a further dose of MMR.
MMR should not be given to women who are pregnant, and pregnancy should be avoided for four weeks after immunisation.[16, 17] However, inadvertent immunisation with a rubella-containing vaccine in early pregnancy is no longer considered an indication for termination of pregnancy. There have been no cases of teratogenic damage from vaccine virus despite intensive surveillance in the US, the UK and Germany.[18]
15.6.2. Precautions
15.6.2. Precautions
Egg allergy, including anaphylaxis, is not a contraindication to MMR. See section 12.6.3 for more information, and section 12.6.2 (external link) for further precautions.
For infants aged under 12 months, please discuss immunomodulatory therapies taken during pregnancy with infant’s mother or specialist, or contact IMAC before administration of MMR0 in an outbreak situation. See section 4.2.5.
15.7. Potential responses and AEFIs
See sections 12.7 and 21.7.
15.8. Public health measures
It is a legal requirement that all cases of mumps be notified immediately on suspicion to the local medical officer of health.
For more details on control measures and notification, refer to the latest version of the 'Mumps' chapter of the Communicable Disease Control Manual.
15.8.1. Diagnosis
15.8.1. Diagnosis
All suspected mumps cases should have diagnostic testing (eg, by buccal swab detection of wild-type mumps virus by PCR or culture) as there are other causes of parotitis other than the mumps virus. See the latest version of the ‘Mumps’ chapter of the Communicable Disease Control Manual for the specimens required for laboratory confirmation of mumps, or discuss these with the local laboratory. See case definition at Communicable Disease Control Manual.
15.8.2. Susceptible contacts
15.8.2. Susceptible contacts
A susceptible contact is anyone born after 1981 who has not had mumps infection or has not been fully vaccinated for their age.
All susceptible contacts should be offered MMR vaccinations. (All vaccinations given should be recorded on the AIR.) There is no increased risk of adverse events after immunisation during the incubation period of mumps or if the recipient is already immune. Immunoglobulin is ineffective after exposure to mumps.
15.8.3. Post-exposure prophylaxis
15.8.3. Post-exposure prophylaxis
Passive immunisation is not effective against mumps. Active immunisation with MMR is not considered effective against incubating infection, but MMR should be offered to susceptible contacts for protection against future exposure.
For detailed information see Management of Cases and Management of Contacts in the mumps chapter of the Communicable Disease Control Manual.
15.9. Variations from the vaccine data sheet
See section 12.9 for variations from the MMR (Priorix) data sheet.
References
References
References
- Ministry of Health. 2012. Mumps in Communicable Disease Control Manual. Wellington. URL: https://www.health.govt.nz/publication/communicable-disease-control-manual (external link). (accessed 10 May 2022)
- Rubin S. 2018. Mumps Vaccines, in Plotkin's Vaccines (7th edition), Plotkin S, Orenstein W, Offit P, et al. (eds). Elsevier: Philadelphia, US.
- American Academy of Pediatrics. 2018. Mumps. in Red Book: 2018 Report of the Committee on Infectious Diseases, Kimberlin D, Brady M, Jackson M, et al. (eds). Elk Grove Village, IL. URL: https://redbook.solutions.aap.org/book.aspx?bookid=2205 (external link). (accessed 3 July 2020)
- Albertson JP, Clegg WJ, Reid HD, et al. Mumps Outbreak at a University and Recommendation for a Third Dose of Measles-Mumps-Rubella Vaccine - Illinois, 2015-2016. MMWR: Morbidity and Mortality Weekly Report, 2016. 65(29): p. 731-4.
- Public Health England. 2017. Laboratory-confirmed cases of measles, mumps and rubella, England: October to December 2016. Infection Report. 11(8): p. 1–5. URL: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/594801/hpr0817__mmr.pdf (external link) (accessed 11 March 2020)
- Centers for Disease Control and Prevention. 2019 Measles Cases and Outbreaks. CDC; 2019 [updated 17 September 2019]; URL: https://www.cdc.gov/mumps/outbreaks.html (external link). (accessed 06 November 2019)
- Demicheli V, Rivetti A, Debalini MG, et al. Vaccines for measles, mumps and rubella in children. Cochrane Database Syst Rev, 2012(2): p. CD004407.
- Davidkin I, Jokinen S, Broman M, et al. Persistence of measles, mumps, and rubella antibodies in an MMR-vaccinated cohort: a 20-year follow-up. Journal of Infectious Diseases, 2008. 197(7): p. 950-6.
- Kontio M, Jokinen S, Paunio M, et al. Waning antibody levels and avidity: implications for MMR vaccine-induced protection. Journal of Infectious Diseases, 2012. 206(10): p. 1542-8.
- Santos EM, Silva e Sa GR, Siqueira MM, et al. Immune response to the mumps component of the MMR vaccine in the routine of immunisation services in the Brazilian National Immunisation Program. Memorias do Instituto Oswaldo Cruz, 2014. 109(3): p. 335-9.
- Carryn S, Feyssaguet M, Povey M, et al. Long-term immunogenicity of measles, mumps and rubella-containing vaccines in healthy young children: A 10-year follow-up. Vaccine, 2019. 37(36): p. 5323-5331.
- Cardemil CV, Dahl RM, James L, et al. Effectiveness of a Third Dose of MMR Vaccine for Mumps Outbreak Control. New England Journal of Medicine, 2017. 377(10): p. 947-956.
- Ogbuanu IU, Kutty PK, Hudson JM, et al. Impact of a third dose of measles-mumps-rubella vaccine on a mumps outbreak. Pediatrics, 2012. 130(6): p. e1567-74.
- Hahné S, Whelan J, van Binnendijk R, et al. Mumps vaccine effectiveness against orchitis. Emerging Infectious Diseases, 2012. 18(1): p. 191-3.
- Australian Technical Advisory Group on Immunisation (ATAGI). 2018. Mumps. in Australian Immunisation Handbook. Canberra. URL: https://immunisationhandbook.health.gov.au/vaccine-preventable-diseases/mumps (external link). (accessed 20 October 2019)
- American Academy of Pediatrics. 2018. Measles. in Red Book: 2018 Report of the Committee on Infectious Diseases, Kimberlin D, Brady M, Jackson M, et al. (eds). Elk Grove Village, IL. p. 537-550. URL: https://redbook.solutions.aap.org/redbook.aspx (external link). (accessed 3 July 2020)
- Strebel P, Papania M, Gastañaduy P, et al. 2018. Measles Vaccine, in Plotkin's Vaccines (7th edition), Plotkin S, Orenstein W, Offit P, et al. (eds). Elsevier: Philadelphia, US.
- Reef SE ,Plotkin S. 2018. Rubella Vaccines, in Plotkin's Vaccines (7th edition), Plotkin S, Orenstein W, Offit P, et al. (eds). Elsevier: Philadelphia, US.