On this page
Post Vaccine Symptom Check (PVSC) is a type of active vaccine safety surveillance. It is used to monitor the safety of vaccines and quantify the frequency of suspected non-serious adverse reactions following vaccination. For example, these could be a sore arm, headaches, or fatigue.
Most non-serious reactions are not captured in the available electronic health data. Therefore, we ‘actively’ seek engagement from consumers by sending a survey to collect data about adverse reactions people may or may not have experienced. We also ask about their overall vaccination experience. The surveys are sent by text message to a random sample of consumers in the days following their vaccination.
Survey responses are anonymised and consumers are not followed up with directly. When a campaign has finished, results are collated into reports that are used for monitoring the safety of the vaccine.
The PVSC does not replace voluntary CARM reporting and any adverse reactions are encouraged to still be reported to the Centre for Adverse Reactions Monitoring (CARM (external link)). If you have questions or concerns about receiving a vaccine or an adverse reaction you may have experienced, please speak to a health care professional.
Key strengths of PVSC include:
- Ability to quantify non-serious adverse reactions
- Encourage participation through direct engagement with consumers
- Provide confidence to the public in the safety of vaccines
- Increase transparency and communicate expectations of a consumer’s vaccination experience
How it works
A sample of the population in New Zealand who receive a vaccine are randomly selected to participate in the survey in the days following their vaccination appointments.
If a consumer is selected to be part of the survey, they are sent an invitation text. This text asks if they or their child have had a side effect after their vaccine. They could respond with “YES”, “NO”, or “STOP”. If they respond “YES” or “NO” they will receive follow up text messages with links one or more surveys. If they do not want to receive these text messages, they can respond “STOP”. If a person does not receive a text message asking if they want to participate in a PVSC survey, it could be that they were not randomly selected, or their contact information is not up to date. The figure below outlines the journey a consumer takes when they have been selected to participate in a PVSC campaign.
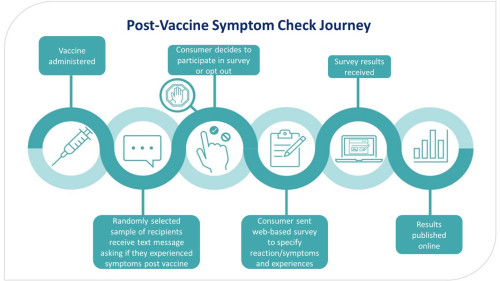
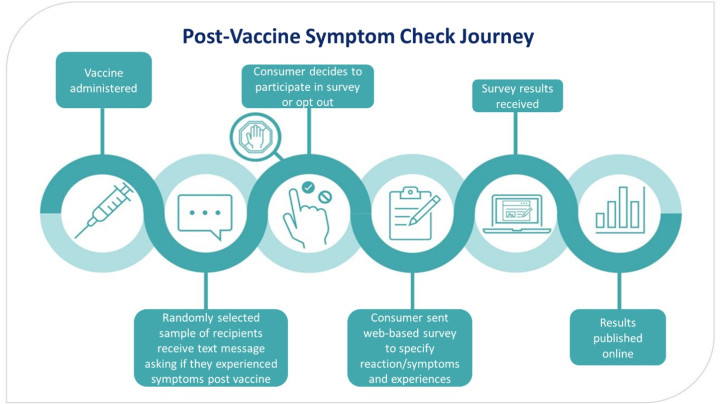
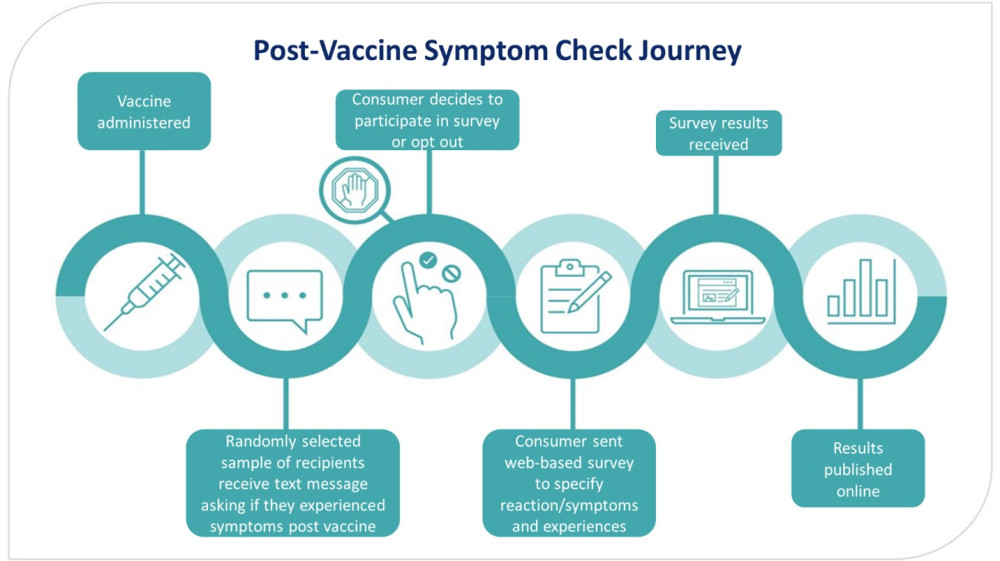
Figure: Post Vaccine Symptom Check journey
The surveys contain questions on early onset adverse reactions, commonly experienced adverse reactions, diagnoses, impacts adverse reactions may have had on daily life, and vaccination experience.
Participating in the survey is optional. If individuals do not want to participate in the survey, they do not have to give a reason, and it does not affect the care people receive.
If an individual decides to participate but change their mind later, they have no obligation to continue and can opt out at any time.
We encourage individuals to report any reactions to a vaccine to the Centre for Adverse Reactions Monitoring (CARM). Reports can be submitted online at the following site: https://pophealth.my.site.com/carmreportnz/s/ (external link). You do not need to be a health professional to submit a report and you do not need to be certain that the reaction was caused by the vaccine. Reporting to CARM does not initiate any personal medical advice or treatment.
If you are concerned about any reactions, you or your child experiences, or if you or your child are unwell, please contact your GP, healthcare provider or Healthline on 0800 611 166.
Survey results
2021-2022 survey results
- 2021-2022 Comirnaty COVID-19 adult vaccine (PDF,200KB) [PDF, 200 KB]
- 2022 Comirnaty COVID-19 paediatric vaccine (PDF 155KB) [PDF, 155 KB]
2023 survey results [PDF, 155 KB]
- 2023 Comirnaty bivalent COVID-19 vaccine (PDF 132KB) [PDF, 132 KB]
- 2023 concomitant Comirnaty bivalent COVID-19 and influenza vaccine (PDF 124KB) [PDF, 124 KB]
- 2023 influenza vaccine (PDF 118KB) [PDF, 118 KB]
2024 survey results [PDF, 155 KB]
Frequently asked questions
How is my privacy protected?
How is my privacy protected?
The information you provide in this survey is confidential and is protected by the Privacy Act 2020 and by the safeguards we have put in place.
We ensure security by:
- Keeping data safe from unauthorised access and use
- Not releasing information that could identify individuals
- Storing health information in the Aotearoa Immunisation Register (AIR), where it is secure and protected
What is done with my responses?
What is done with my responses?
- Information collected from the survey will be summarised and reported on once we have enough data.
- Health New Zealand | Te Whatu Ora will analyse survey responses and investigate if there are any trends, for example rates of specific events in different ethnic groups, age ranges and/or genders. They will also investigate the response rates from different groups of people to ensure health equity.
- Health New Zealand | Te Whatu Ora will not follow up on any survey responses. If you or your child feel unwell or are concerned about your or your child’s reactions to a vaccine, please contact your healthcare provider.
How did Health New Zealand | Te Whatu Ora get my mobile number?
How did Health New Zealand | Te Whatu Ora get my mobile number?
- The Post Vaccine Symptom Check uses the mobile numbers provided in the Aotearoa Immunisation Register (AIR).
- As this is a complementary method to improve safety assessments of vaccines in New Zealand, it does not require you to register, like how your GP might follow up via text with results of a lab test.
- If you wish to opt out of participating in the survey, you can do so at any time by replying STOP.
- If you have previously declined to be contacted by the information provided in the AIR, you will not be contacted for the Post Vaccine Symptom Check.
Is it free to reply to the text message?
Is it free to reply to the text message?
Yes, it is free to reply to the text message. You will need access to the internet to be able to complete the survey. This will use data if you are not connected to Wi-Fi.