On this page
What information we collect
NZCR collects data for almost all malignant tumours (invasive and in-situ) first diagnosed in New Zealand. For exclusions please see the 'What is not collected' section.
An overview of the information to be reported is contained in the Cancer Registry Regulations 1994 (external link). This data is collated and coded by a specialised team of cancer coders at NZCR. NZCR collects detailed pathological information about each tumour, as well as key demographic information to ensure that each new cancer is recorded only once in incidence statistics.
Data in the NZCR system includes:
In relation to the person to which the report relates:
- name
- NHI number
- sex
- address
- ethnicity.
In relation to the tumour to which the report relates:
- date of diagnosis
- site of primary cancer (or secondary site if primary unknown)
- type of cancer test (basis of diagnosis)
- morphology
- grade
- staging information
- site-specific information (eg, Breslow's thickness for melanoma, ER/PR status for breast cancer)
Cancer incidence reporting
Not all tumours collected in the NZCR are included in incidence reporting.
In-situ cancers
In-situ cancers
In-situ cancers (localised lesions that have not invaded beyond the basement membrane) are recorded in NZCR but are excluded from incidence reporting.
Multiple tumours
Multiple tumours
Incidence counts are based on the number of primary tumours rather than the number of individuals with cancer. The NZCR database records multiple primary cancers in the same person, of which only some are counted for incidence purposes, according to the rules of the International Agency for Research on Cancer (IARC) and the International Association of Cancer Registries (IACR).
In brief, these rules are:
- Recognition of the existence of two or more primary cancers does not depend on time.
- A primary cancer is one that originates in a primary site or tissue and is thus neither an extension, a recurrence nor a metastasis (transfer of cancerous cells to other parts of the body) of a pre-existing tumour.
- Only one tumour shall be recognised in an organ or pair of organs or tissue unless of a different histology. Some groups of codes are considered to be a single organ for the purposes of defining multiple tumours.
- A cancer with a different histology in the same organ is counted as a new tumour.
An example of a multiple tumour is invasive ductal carcinoma of the right breast in a woman who has had a previous diagnosis of invasive ductal carcinoma of the left breast. Both cancers are recorded as separate registrations in NZCR, but only the cancer in the left breast (diagnosed first) is counted in incidence statistics.
Tumour staging
Staging information is collected where available. Because pathology reports are the main source of data this information is most complete for tumours where the primary treatment is surgical (eg, melanoma, breast and colorectal cancers).
SEER summary staging
SEER summary staging
NZCR uses SEER summary staging to record the extent of disease at diagnosis.
Summary staging refers to the classification of a cancer case into a broad category (in-situ, localised, regional extension and distant metastases), representing the extent of involvement of the tumour as determined using all diagnostic and therapeutic evidence available at the end of the first course of therapy; or within four months of the date of diagnosis, whichever is earlier.
The staging system was developed by the Surveillance Epidemiology and End Results (SEER) program of the National Cancer Institute of the USA, specifically for use by cancer registries. The SEER system can be applied to all types of tumours.
It's useful because of its international comparability and stability over time, and the simplicity of its application in the absence of complete data. The SEER summary staging manual, 2018 (external link) is available from the SEER website.
NZCR has adapted this manual for use in our system. NZCR clinical coders enter the extent of disease code using information from pathology reports, hospital event data (external link)and the New Zealand Mortality Collection (external link).
TNM classification of malignant tumours
TNM classification of malignant tumours
This classification was introduced by the Union for International Cancer Control (UICC) and the American Joint Committee on Cancer (AJCC).
This classification defines the primary site by extent, degree of nodal disease and presence or absence of distant metastasis. It is used only for the staging of solid tumours (it is not used for staging haematological malignancies).
- T describes the size and local extent of the tumor
- N describes the extent of nodal involvement
- M indicates whether or not there are metastases
Two classifications are described for each site, clinical (pre-treatment clinical classification) and pathological (post-surgical histopathological classification). TNM staging is only recorded in the NZCR if it is available on the pathology report.
What information we don't collect
NZCR does not record basal cell and squamous cell cancers of the skin unless they arise in the skin of the genitalia. The registration of these cancers was discontinued in 1958 due to resource considerations. Benign neoplasms, including those situated in the brain, are also not registered.
NZCR is not a clinical management system and does not collect treatment information. Some data on treatment may be found in other Ministry of Health collections such as National Minimum Dataset (NMDS) (external link), National Non-Admitted Patient Collection (external link) and the Pharmaceutical Collection (external link).
Data sources
Before the introduction of the Cancer Registry Act and Regulations in 1994, the collection of morphology information was limited, as most registration data was obtained from hospital discharge data rather than histological reports.
Since this legislation came into effect detailed morphology data has been collected from pathology reports.
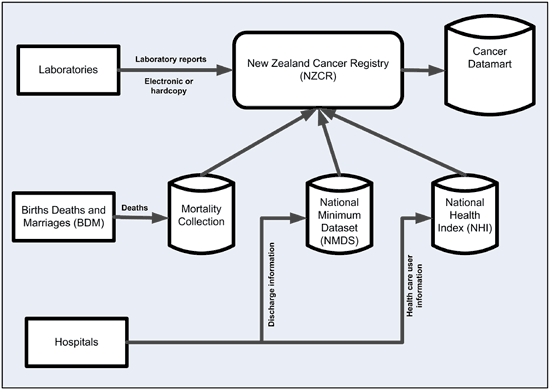
Laboratories
Laboratories
Laboratories are the primary source of cancer data for the NZCR. They are required by law to report any reportable new diagnosis of cancer. Since 2003 most pathology reports have been sent via the secure electronic link, Healthlink.
Any reports still received in paper form are scanned into NZCR. All pathology reports received since 2002 are stored electronically in the secure NZCR system.
Hospital data
Hospital data
Cancer registry records are checked against hospital discharge data on the National Minimum Dataset (NMDS) (external link)
Mortality Collection
Mortality Collection
Records from the Mortality Collection are used to identify missing cases and to provide follow up details.