On this page
- Section A – infectious diseases notifiable to a medical officer of health and local authority
- Campylobacteriosis
- Cholera
- Cryptosporidiosis
- Giardiasis
- Hepatitis A
- Legionellosis
- Listeriosis
- Meningoencephalitis – primary amoebic
- Salmonellosis, typhoid and paratyphoid fever
- Shigellosis
- Yersiniosis
- Section B – infectious diseases notifiable to the medical officer of health
- Anthrax
- Arboviral infection other than yellow fever
- Avian influenza and non-seasonal influenza (last updated 12 December 2024)
- Brucellosis
- Creutzfeldt-Jakob disease and other spongiform encephalopathies
- Cronobacter species (formerly Enterobacter sakazakii invasive disease)
- Diphtheria
- Haemophilus influenzae type b invasive disease
- Hepatitis B infection
- Hepatitis C, recent infection
- Hepatitis D
- Hepatitis E
- Hydatid disease
- Invasive pneumococcal disease
- Leprosy
- Leptospirosis
- Malaria
- Measles
- Middle East respiratory syndrome (MERS)
- Mumps
- Neisseria meningitidis invasive disease
- Novel coronavirus capable of causing severe respiratory illness
- Pertussis
- Plague
- Poliomyelitis
- Q fever
- Rabies and other lyssaviruses
- Rheumatic fever
- Rickettsial disease
- Rubella
- Rubella – congenital
- Severe acute respiratory syndrome (SARS)
- Tetanus
- Tuberculosis, active (new case, reactivation)
- Verocytotoxin- or Shiga toxin-producing Escherichia coli (VTEC/STEC)
- Viral haemorrhagic fevers
- Yellow fever
- Section C – infectious diseases notifiable to the medical officer of health without identifying information of patient or deceased person
- Acquired immunodeficiency syndrome (AIDS)
- Gonorrhoeal infection
- Human immunodeficiency virus (HIV) infection
- Syphilis
- Diseases notifiable to the medical officer of health (other than notifiable infectious diseases)
- Cysticercosis
- Trichinosis
Appendix updated in December 2024. A description of changes can be found at Updates to the Communicable Disease Control Manual.
Section A – infectious diseases notifiable to a medical officer of health and local authority
Campylobacteriosis



Campylobacteriosis
Notes:
- All species of Campylobacter should be notified. Diagnostic laboratories may choose to identify further than genus level but should refer isolates for confirmatory speciation to the Enteric Reference Laboratory at ESR.
- Please keep samples for two weeks in case culture is required for typing for public health purposes in an outbreak investigation.
Cholera



Notes:
- The notifiable condition is disease due to toxin-producing Vibrio cholerae O1 or O139.
- Vibrio should be identified to the species level.
Cryptosporidiosis



Giardiasis



Hepatitis A



Notes:
- In the absence of HAV vaccination in the preceding 12 weeks.
- Patient serum and/or faecal specimens (preferably both) should be sent to Specimen Reception at ESR Kenepuru Science Centre, Porirua for HAV genotyping by the ESR Enteric, Environmental and Food Virology Laboratory.
Legionellosis



Notes:
- Improved PPV obtained by using more than one test type.
- Validated tests only.
- One or more elevated Legionella species serology titres of ≥ 512 tested using pooled antigen at a reference laboratory is considered suggestive evidence for probable case.
- Legionella Reference Lab, ESR, should report all results.
Listeriosis



Meningoencephalitis – primary amoebic



Notes:
- For further information on referring samples refer to the CDC website.
- NAAT testing is not available in New Zealand but samples can be sent to Australia. Discuss laboratory testing with the Institute of Environmental Science and Research (ESR).
Salmonellosis, typhoid and paratyphoid fever



Notes:
- Salmonella serology may provide evidence of past infection but is not useful for diagnosis of acute illness. Requests for Salmonella serology should be replaced by blood cultures if the patient has a febrile illness.
- Salmonella Paratyphi B var Java infections should still be notified as Salmonella cases rather than cases of Paratyphi.
Shigellosis



Note:
- While NAAT may be used for screening, a positive NAAT does not meet the criteria for laboratory confirmation.
Yersiniosis



Section B – infectious diseases notifiable to the medical officer of health
Anthrax



Arboviral infection other than yellow fever



Note:
- Please e-notify results, including those done overseas.
Avian influenza and non-seasonal influenza (last updated 12 December 2024)
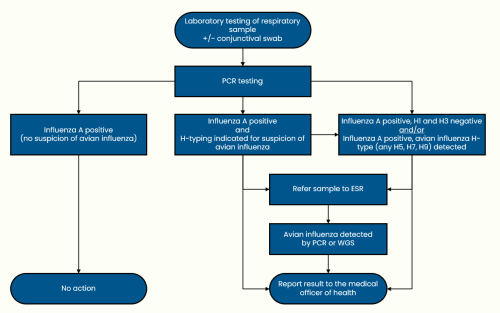
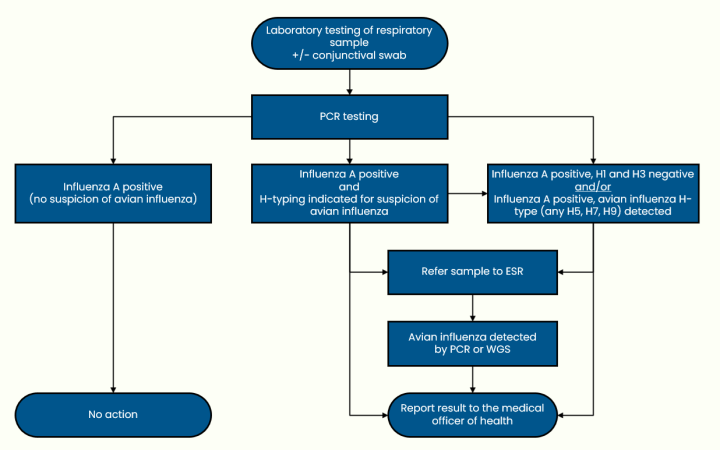
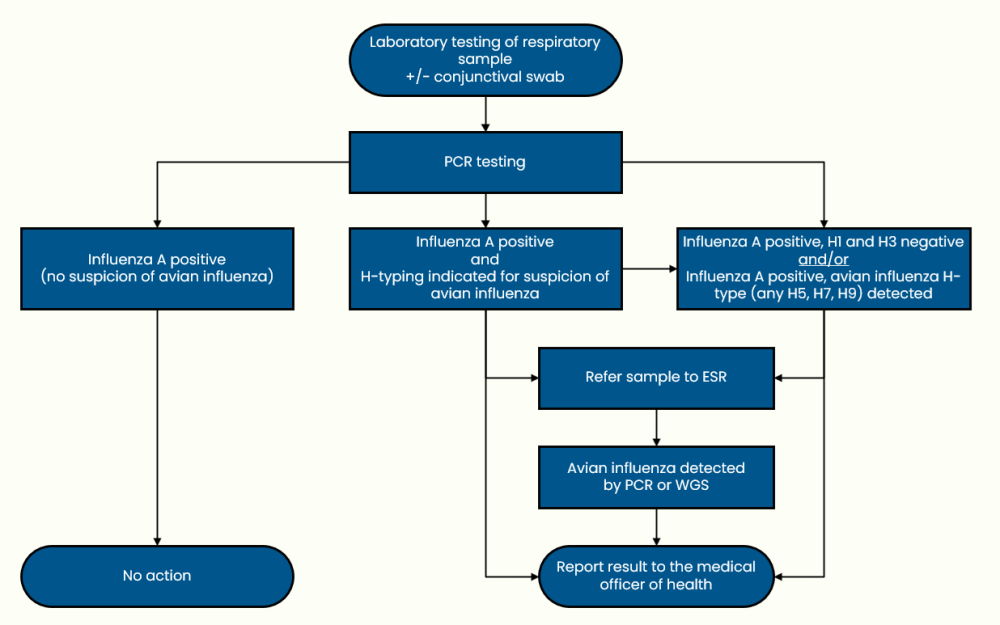
Brucellosis



Note:
- Consider the possibility of cross-reactivity.
Creutzfeldt-Jakob disease and other spongiform encephalopathies



Cronobacter species (formerly Enterobacter sakazakii invasive disease)



Diphtheria



Note: The diagnosis of diphtheria is primarily a clinical one. Tox-containing, nontoxigenic isolates have been described.
Haemophilus influenzae type b invasive disease



Hepatitis B infection



Note:
- Recent immunisation with HBV vaccine may also result in detectable HBsAg for a short period of time. Since laboratories do not necessarily have access to this information, all results consistent with possible Hepatitis B infection should be reported to the medical officer of health.
Hepatitis C, recent infection



Hepatitis D



Note:
- Report all positive antibody results so the medical officer of health can ensure that follow up testing is performed.
Hepatitis E



Notes:
- A positive IgM alone should be confirmed by either anti-HEV IgG or total antibodies or NAAT testing.
- Report all positive antibody results so the medical officer of health can ensure that follow up testing is performed.
Hydatid disease



Invasive pneumococcal disease



Leprosy



Note:
- Where confirmed by sequencing or validated species-specific PCR.
Leptospirosis



Malaria



Note:
- Microscopy of P. knowlesi may resemble P. malariae or P. falciparum. If the patient has travelled to SE Asia or has severe malaria and has been diagnosed with P. malariae or P. falciparum, further testing by NAAT may be needed to confirm/exclude P. knowlesi.
- If possible, this result should be confirmed by microscopy or NAAT.
Measles



Note:
- Recent immunisation with MMR may also result in detectable anti-measles IgM, a significant increase in anti-measles IgG or a positive NAAT. Since laboratories do not necessarily have access to this information, all results consistent with possible measles infection should be reported to the medical officer of health. Further testing for vaccine strain will be arranged by the medical officer of health when appropriate.
Middle East respiratory syndrome (MERS)



Mumps



Note:
- Recent immunisation with MMR may also result in a positive NAAT. Since laboratories do not necessarily have access to this information, all results consistent with possible mumps infection should be reported to the medical officer of health. Further testing for vaccine strain will be arranged by the medical officer of health when appropriate.
Neisseria meningitidis invasive disease



Notes:
- Arrange for NAAT testing on CSF if cultures are sterile, so that amplification product can be further characterised by NRL.
- Meningococcal conjunctivitis should be notified to the medical officer of health because of the potential for invasive disease in contacts of the case.
- Only if clinical details provided of invasive meningococcal disease.
- Meningococci isolated from genital swabs are not associated with systemic disease (except for rare neonatal meningitis in babies born to colonised mothers) and need not be reported to the medical officer of health.
- If the primary laboratory is able to distinguish serogroup, this information is useful for the local PHU when planning vaccination of contacts.
Novel coronavirus capable of causing severe respiratory illness
Testing for a novel coronavirus will likely be similar to the testing for COVID-19.
Pertussis



Note:
- Serology should only be requested after consultation between the medical officer of health and the microbiologist.
Plague



Poliomyelitis



Note:
- Enteroviral PCR performed on CSF, faeces or respiratory samples may detect an enterovirus potentially including poliovirus. Unless PCR amplification product has been further characterised because of the clinical scenario, positive enterovirus PCR results need to be notified to the medical officer of health.
Q fever



Notes:
- The date on which the patient first became ill is required to interpret Q fever serology. A serological response will be detected 10–30 days after onset of illness.
- Recent immunisation may also result in a significant increase in anti-C. burnetii antibodies. Since laboratories do not necessarily have access to this information, all results consistent with possible recent C. burnetii infection should be reported to the medical officer of health.
- Report all positive antibody results so the medical officer of health can ensure that follow up testing is performed.
Rabies and other lyssaviruses



Note:
- It is unlikely that laboratory testing for these viruses is performed within New Zealand at this time. Specimens from suspected cases would be referred to overseas laboratories.
Rheumatic fever
The diagnosis is clinical.
Detection of pharyngeal S. pyogenes or changing streptococcal serology does not make the diagnosis.
No action is required for laboratories.
Rickettsial disease



Notes:
- Report all positive antibody results so the medical officer of health can ensure that follow up testing is performed.
- If specific type of Rickettsial species by NAAT is not available, then serology should be used to determine if the case is murine typhus, spotted fever or scrub typhus group.
The following serological tests are available at LabPlus:
- Rickettsia typhi for murine typhus
- Orientia tsutsugamushi for scrub typhus group
- Rickettsia conorii for tick typhus group.
The following serological tests are available at Waikato Hospital:
- Rickettsia typhi IgG and IgM
- Spotted fever group IgG and IgM.
Rubella



Note:
- Recent immunisation with the measles-mumps-rubella vaccine (MMR) may also result in detectable anti-rubella IgM or a significant increase in anti-rubella IgG. Because laboratories do not necessarily have access to this information, all results consistent with possible rubella infection should be reported to a medical officer of health.
Rubella – congenital



Note:
- That is, rubella titre that does not drop at the expected rate of a twofold dilution per month.
Severe acute respiratory syndrome (SARS)



Tetanus
The diagnosis is clinical.
Neither culture of the organism nor presence of antibodies to the toxin is proof of disease.
No action is required for laboratories.
Tuberculosis, active (new case, reactivation)



Note:
- Samples should be collected for mycobacterial culture, if not already done.
Latent tuberculosis
Latent tuberculosis (LTBI) is only reported when there is a decision to treat, and with permission of the case. Therefore, no action is required by laboratories.
Verocytotoxin- or Shiga toxin-producing Escherichia coli (VTEC/STEC)



Viral haemorrhagic fevers
Laboratory testing for these viruses is not performed at this time within New Zealand.
Specimens from suspected cases would be referred to overseas laboratories. Discuss laboratory testing with ESR.
Yellow fever



Notes:
- Yellow fever serology gets sent to Westmead, Sydney.
- Yellow fever PCR is done at LabPlus.
Section C – infectious diseases notifiable to the medical officer of health without identifying information of patient or deceased person
Acquired immunodeficiency syndrome (AIDS)
AIDS is a clinical syndrome. No action is required by laboratories.
Gonorrhoeal infection



Notes:
- It is recommended that culture negative, reactive NAAT results on specimens from extra-genital sites be confirmed by supplementary testing, using a different nucleic acid target, before reporting depending on the test method used.
- Given difficulties in the implementation of gonorrhoea and syphilis notification system, the reporting to medical officer do not yet apply to the laboratory notification process. Laboratories will be advised when this issue is solved.
Human immunodeficiency virus (HIV) infection



Notes:
- Whether positive or negative, the result of the first NAAT/viral load test performed must be notified for surveillance purposes. Subsequent new positive results on an individual for whom all previous results have been negative, also require notification. Subsequent results (positive or negative) on the same individual do not require notification. (Note: All subsequent results may be requested separately for other purposes, such as cascade of care monitoring, but this would not be done through the e-notification system.)
- Any known cases previously diagnosed in New Zealand and having a viral load test, will be filtered by the AEG.
Syphilis



Note:
- Given difficulties in the implementation of gonorrhoea and syphilis notification system, the reporting to medical officer do not yet apply to the laboratory notification process. Laboratories will be advised when this issue is solved.
Diseases notifiable to the medical officer of health (other than notifiable infectious diseases)
Cysticercosis
- Cysticercosis is caused by the larval stage of T. solium after ingestion of eggs (rather than encysted larvae) from contaminated food, water or via faecal-oral autoinoculation.
- Stool examinations can be performed; however, eggs are typically not found, since the majority of people diagnosed with cysticercosis do not have a viable T. solium tapeworm in their intestines.
- Serology is available through reference laboratories for patients with suggestive radiological findings. Occasionally, the diagnosis of extraneural cysticercosis is made by finding a larval scolex in an excisional biopsy of a skin or muscle lesion.



Taeniasis



Notes:
- If cysticercosis is also suspected, possible use of serology should be discussed with the clinical microbiologist.
- It is not possible to differentiate the eggs of T. solium from the beef tapeworm T. saginata. Identification to species level requires examination of proglottid segments passed in the stool.
Trichinosis



Notes:
- Muscle biopsy for histology collected at least 10 days and ideally ~4 weeks after infection.
- Eosinophilia is supportive but not diagnostic.